Research progress in the fabrication and application of rare-earth single-atom photocatalysts
-
摘要: 单原子光催化剂具有增强光捕获、电荷转移和光催化的表面反应等方面的优势,已成为目前光催化领域非常活跃的研究方向。稀土元素电子结构独特,表现出优越的光学、电学等方面的性质,被广泛应用于催化科学研究。将稀土金属的尺寸降低到单原子尺度,对于提高稀土元素的光催化效率及其利用效率非常重要。本文对稀土单原子光催化剂进行了概述,重点分析了目前稀土单原子光催化剂的制备方法和稀土单原子光催化剂的应用,并对稀土单原子光催化剂面临的挑战和发展方向进行了展望。Abstract: With distinct advantages of enhanced light capture, high charge transfer rate and fast surface reactions in photocatalytic systems, single-atom photocatalysts have become one of the most active research directions in the photocatalytic field. Rare earth elements have a unique electronic structure, exhibit superior optical, electrical and other properties, and are widely used in photocatalytic research. Reducing the size of rare earth metals to the single-atom scale is very important for improving photocatalytic efficiency and utilization efficiency. The rare earth single-atom photocatalysts are summerized, focusing on the current preparation method of rare earth single-atom photocatalysts and the application of rare earth single-atom photocatalysts. The challenges and development directions of rare earth single-atom photocatalysts are discussed.
-
Keywords:
- rare earth single-atom photocatalysts /
- photocatalysis /
- review
-
光催化是一种绿色化学技术,是通过光触发实现化学反应的高级氧化技术,是解决环境污染以及能源危机最有潜力的方法之一[1-5],光催化的反应效率较低,因而较难实现工业推广应用。因此,设计高效的光催化剂材料非常关键[6-10]。近十余年,研究者一直致力于研究并探索具有优异光捕获能力、高电荷载流子分离效率的光催化剂。光催化技术在能源和环境领域有着广阔的应用前景[11-16]。
近年来,稳定在适当载体上的单个或孤立的金属原子形成的催化剂(即单原子催化剂(SAC)[17-21])的研究备受关注[22-26]。单原子催化剂通常具有独特的催化性能[27-32],同时还可以通过反应中间体限制原子级吸附,实现不同的催化反应途径[32-42]。由于具有均匀分布的不饱和配位位点,单原子催化剂还为实现多相催化和均相催化之间的跨越提供了一个模型。单原子催化剂具有超高的原子利用效率(约为100%),大大降低了催化金属的使用量。
近期研究表明,单原子催化剂(SAC)在提高光催化活性方面具有独特的优势[43-46]。从光催化的角度,由于金属单原子和载体之间相互作用,将单个的原子嵌入到载体中可以改变催化剂的电子结构和能带结构,可据此调节催化剂的电荷转移动力学和光吸收行为。另外,单原子光催化剂复合不同的金属后会呈现不同的表面结构,通过调节不同的表面结构可以增强光催化剂对反应物的吸附和活化能力[47-51]。
1 稀土单原子光催化剂
稀土单原子催化剂(RE-SAC),即以单原子稀土元素为活性中心制备的催化剂。由于稀土单原子具有独特的电子、光学和磁性等方面的性质,因此被广泛应用于许多重要的科学研究和工业制造等领域[52-55],很多稀土元素(RE)因具有多壳层电子结构而被用于多相催化反应[56]。将稀土金属缩小到单原子尺度是提高金属利用效率的有效策略。同时,稀土金属单原子尺度可使其具有独特的催化性能。
在多相催化过程中,稀土元素通常作为促进剂或载体,这是由于其具有独特的轨道屏蔽效应,即f轨道的电子不参与化学键的形成或与配体有显著的轨道重叠[57-59]。这种f轨道和其电子可与催化剂载体材料中的阴离子形成强配位键,为稀土单原子催化剂的形成提供可能[60]。此外,稀土元素通常表现出Lusic酸性,可与具有高Lusic碱度的阴离子基团发生强烈配位,对强化活化反应物具有非常显著的作用[61-65]。
稀土单原子掺杂进入半导体可以调节半导体光催化剂晶格、能级结构和表面吸附性能,从而提高光催化剂的光学吸收能力、光生载流子的迁移和分离速率和对反应物的吸附能力[11]。此外,镧系元素(Ln)具有明确的4f→4f跃迁电子轨道以及很高的光化学稳定性,在紫外可见到近红外区域具有明显的发光光谱[66]。因此,RE或镧系元素经常被用于合成荧光/磷光材料和上转换光催化材料[67-71]。此外,由于其电子性质和较大的半径,稀土原子通常形成具有至少4个配体的稳定高配位结构[72]。负载在不同载体上的RE-SAC通常可以表现出不同的性能,包括活性、选择性和稳定性等[73-75]。
2 稀土单原子光催化剂的合成方法
常见的RE-SAC合成方法主要有浸渍法、高温固相合成法、化学沉积法、水热处理法和溶胶-凝胶法等。
2.1 浸渍法
浸渍法即将固体粉末或一定形状及尺寸的已成型的固体(载体或含主体的催化剂)浸泡在含有活性组分的可溶性溶液中,接触一定的时间后分离残液,活性组分以离子或化合物的形式附着在固体上[76]。浸渍法是目前催化剂工业生产中广泛应用的方法。WANG等[77]将原子分散的Pt以0.05%(w/w)负载在CeO2纳米颗粒表面合成单原子Pt/CeO2,而纳米团簇Pt/CeO2的负载量可达2%(w/w)。在CO2还原过程中,单原子Pt/CeO2的反应活性高于纳米团簇Pt/CeO2。PARNICKA等[78]使用TiO2-NTs合金作为阳极材料,以两步阳极氧化的方法制备了多种稀土金属修饰的TiO2-NTs。两步阳极氧化即首先合成阳极TiO2-NTs,然后将NTs浸入相应的稀土金属前体溶液中,再掺杂稀土金属离子[79]。
2.2 高温固相合成法
高温固相合成法通常是将2种及以上的金属化合物通过研磨等方式使其混合均匀,再利用马弗炉等仪器将充分研磨均匀的混合物高温加热[80]。GAN等[81]将四水醋酸铈(Ⅲ)和醋酸金(Ⅲ)球磨,并在空气中进一步煅烧,制备得到质量高达1.025 kg的单原子Au1/CeO2催化剂;采用相同的方法,以不同的前驱体合成了Ir1/CeO2、Au1/NiO和Au1/ZnO催化剂,验证了该方法的适用性。LIU等[82]通过球磨法合成的Y1/NC和Sc1/NC催化剂具有优异的电催化还原N2和CO2性能。XU等[21]通过球磨和煅烧在(CeZrHfTiLa)Ox(HEFO)上合成了单原子Pd,该催化剂表现出较高的低温CO氧化活性和优异的抗热降解和水热降解性能。
JIANG等[83]通过浸渍和在空气气氛下的进一步煅烧,合成了在低温下表现出良好的CO氧化性能的Pd-CeO2。除了金属氧化物基质外,该研究组还通过相同的方法制备了许多固定在碳材料上的单原子催化剂。LI等[84]通过将Ce3+浸入十六烷基三甲基溴化铵(CTAB)、过硫酸铵(APS)和吡咯的黑色聚合沉淀物中,在氮气气氛下进行高温处理,在碳纳米线上合成了高度分散的铈原子。此外,该研究组采用相同的方法制备的Fe-N-C材料在氧还原反应(ORR)中表现出高活性[85]。LI等[86]采用浸渍煅烧法在2D-TiO2纳米片表面修饰了稀土单原子(La,Er),通过AC-HAADF、STEM和XAS等表征证实了稀土单原子的形成,并通过活性测试证明了单原子在中间产物的调节和彻底矿化过程中具有重要作用。
CHEN等[65]通过一步煅烧法设计并制备了O/La-CN光催化剂,证明了La单原子的形成,阐明了La单原子的4f和5d轨道以及La-N的d轨道的杂交原子形成了电荷转移通道,并表明该催化剂以La-N电荷转移桥作为光催化CO2反应的活性中心。DEREVYANNIKOVA等[87]将Ce(NO3)3与Pt(NO3)4溶液混合,然后向混合物中添加1 mol/L KOH以形成沉淀,随后在空气中进行煅烧得到Pt-CeO2。ZHU等[88]采用相同的方法制备了Ce-SAS/HPNC催化剂。
2.3 化学沉积法
化学沉积是利用一种合适的还原剂使镀液中的金属离子还原并沉积在基体表面上的化学还原过程。化学沉积具有化学气相沉积和液相沉积2种模式。在各种沉积技术中,化学沉积产生稳定、成本低、具有良好的再现性[89]。YE等[90]以三甲基(甲基环戊二烯基)-铂(Pt)(Ⅳ)作为铂前体,臭氧作为氧化剂,在CeO2纳米棒上制备原子分散的铂。WANG等[91]采用该方法制备了单原子Pt1/CeO2。WANG等[92]将TiO2沉积在介孔SBA-15分子筛上,并在催化剂表面沉积稀土金属钕(Nd),得到Nd-TiO2-SBA-15光催化剂。WADA等[93]以CeO2作为载体,Cu(NO3)2·3H2O为前驱体,Na2CO3为沉淀剂,通过水热法制备了单原子Cu-CeO2,该催化剂在电化学CO2还原过程中对甲烷表现出高选择性,法拉第效率为58%。
2.4 水热法和溶剂热法
水热法和溶剂热法是通过将反应试剂置于一个较密闭的反应环境中,以水或其他液体充当溶剂,粉体经溶解和再结晶制备材料的方法。在高温高压条件下,相对无规则运动加剧,促进溶质相互接触,增加反应几率,提高产品纯度,而水热(溶剂热)反应可以减缓因反应加剧导致的纳米颗粒团聚的现象[94]。KASUGA等[95]开发了一种利用水热法合成1D NSs特别是高比表面积NTs的新方法,并证明通过加入金属、有机或无机材料,可以极大地提高其光电性能。LI等[96]通过水热合成法和原位合成法相结合成功构建了Eu3+单原子掺杂CdS/InVO4 Ⅱ型异质结。该复合材料在可见光下具有优异的光催化CO2还原活性。ZHU等[97]采用一锅水热法成功制备了具有混合晶相的BiVO4微球,并用Sm3+调整BiVO4微球形态和光催化活性。LIU等[98]采用溶剂热-光沉积的方法制备了La/MoO3-x光催化剂,成功地将单La原子锚定在MoO3-x中,证明了单个La原子可以显著优化局部电子性质,并且吸附在O2c-La-O2c位点上的氮分子可以被激活。
2.5 溶胶-凝胶法
溶胶-凝胶法是相较于固相反应而言更加简便且催化剂尺寸可控的方法。在制备溶胶的过程中,将溶质充分溶解在合适的溶剂中,静置,溶液内某些成分会逐渐凝聚在一起而形成溶胶,再进一步静置后形成凝胶。控制温度,使这些凝胶发生水解缩聚反应,成功制备得到目标产物[99]。SOWIK等[100]采用简单的溶胶-凝胶法制备了一系列稀土修饰的ZnO量子点,系统研究了镧系元素的种类(Eu、Er、Tb、Yb、Ho、La)和数量(0.09~0.45 mmol)对ZnO/RE量子点光学性质、结构和光催化活性的影响。
2.6 其他方法
WANG等[101]采用半夹层稀土金属催化剂,实现了含氧、含硫、含硒、含氮和含磷等一系列α-烯烃的内聚反应,及其与乙烯的共聚反应。该方法有效地制备出新的杂原子功能化聚烯烃家族。
HU等[102]利用级联锚固策略在多孔碳基板上负载了稀土Pr单原子,其金属负载高达5.07%(w/w)。该复合催化剂在电化学CO2还原反应过程中表现出优异的催化性能,并对CO具有很高的选择性。
WU等[103]采用硬模板法合成稀土单原子Ce,其中CeO2是在气体迁移过程中引入的。在流动的氮气中,在1 150 ℃时,CeO2的表面Ce原子最初蒸发,形成可能的挥发性Ce物种,其可被缺陷的富氮多孔碳运输和捕获,形成原子分散的Ce-SAS催化剂。
WEI等[104]通过拓扑引导法和溶剂热反应法成功设计了2种结构稀土酰胺功能化MOFs。2种MOFs均显示出理想的包含双笼状和通道状孔隙几何形状的拓扑网络。
JEYASEELAN等[105]通过热液法成功制备了稀土金属(RE)基MOFs,即La和Ce修饰的苯-1, 3, 5-三羧酸(BTC),La@BTC和Ce@BTC MOFs是最有前途的吸附剂材料,尤其在氟吸附方面。该研究采用多种表征手段对RE基MOFs的表面性质和性能进行了表征。
3 稀土单原子光催化剂的应用
3.1 光催化还原CO2
光催化还原CO2转化为有价值的化学产品(如CO、CH4和CH3OH等)是目前光催化领域的研究热点。
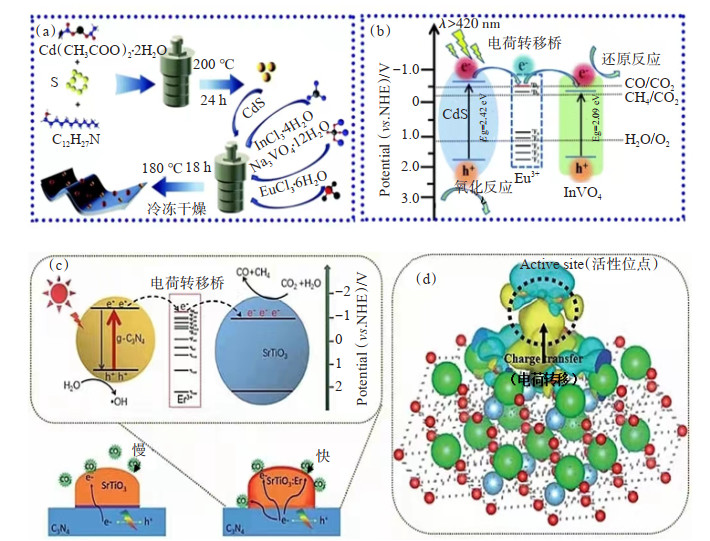
LI等[96]将水热合成法和原位合成法相结合成功构建了Eu3+单原子掺杂CdS/InVO4 Ⅱ型异质结(图 1(a))。活性测试结果表明,该复合材料在可见光下具有优异的光催化CO2还原活性,并且描述了CO2还原机理(图 1(b)):当Eu3+存在时,电子可从CdS转移到InVO4。实验结果显示,CdS/InVO4: Eu3+的CO产率分别是纯InVO4的2.4倍,纯CdS的1.9倍;CH4产率分别是InVO4的5.3倍,CD的10.6倍。这表明单原子Eu3+修饰InVO4既达到了活化CO2分子的目的,也促进了界面之间的电荷分离,从而提高了催化剂的光催化性能。
![]() 图 1 稀土单原子光催化剂的合成及机理:(a)CdS和CdS/InVO4:Eu3+的合成[96];(b)可见光照射下光催化和光还原CO2过程的机理[96];(c)Eu3+作为ET桥促进电荷转移机理[60];(d)Eu3+作为ET桥促进电荷分离机理[60]Figure 1. Synthesis and mechanism diagram of rare earth monoatomic photocatalyst: (a) Synthesis diagrams of CdS and CdS/InVO4: Eu3+ [96]; (b) Proposed mechanism diagram of photocatalysis and the photoreduction of CO2 process under visible light irradiation[96]; (c) Mechanism for Eu3+ as ET bridge to promote charge transfer[60]; (d) Mechanism of Eu3+ as ET bridge to promote charge transfer separation[60]
图 1 稀土单原子光催化剂的合成及机理:(a)CdS和CdS/InVO4:Eu3+的合成[96];(b)可见光照射下光催化和光还原CO2过程的机理[96];(c)Eu3+作为ET桥促进电荷转移机理[60];(d)Eu3+作为ET桥促进电荷分离机理[60]Figure 1. Synthesis and mechanism diagram of rare earth monoatomic photocatalyst: (a) Synthesis diagrams of CdS and CdS/InVO4: Eu3+ [96]; (b) Proposed mechanism diagram of photocatalysis and the photoreduction of CO2 process under visible light irradiation[96]; (c) Mechanism for Eu3+ as ET bridge to promote charge transfer[60]; (d) Mechanism of Eu3+ as ET bridge to promote charge transfer separation[60]CHEN等[60]构建了Er3+单原子复合光催化剂,并采用高角环形暗场像-扫描透射电子像(HAADF-STEM)技术研究了STO: Er3+/CN复合材料中Er单原子的形成。该研究并未局限于将稀土离子作为光转换剂的传统观点,而是以Er3+作为ET桥促进电荷分离(图 1(c)和图 1(d)),实现了光生电子从CN导带到STO的高效转移,增强了界面电荷分离效率,表明Er3+表面具有电荷分离和CO2活化的双重功能,促进了CO2的光催化活性。实验结果表明,STO: Er3+/CN在可见光照射下表现出比原始g-C3N4和STO/CN更高的光催化性能。此外,HAN等[106]构建了Zn2GeO4: Er3+/g-C3N4复合光催化剂,证明了原位合成的Zn2GeO4: Er3+/g-C3N4不仅更有利于Zn2GeO4与g-C3N4的紧密结合,也更容易在g-C3N4上锚定稀土原子。在可见光辐照下,Zn2GeO4: Er3+g-C3N4光催化体系的催化效率比没有牺牲剂的纯g-C3N4提高了5倍以上。该研究利用了Er3+单原子对于CO2分子活化和4f能级作为电子传递桥的双重作用,将单原子催化和异质结相结合,为提高光催化活性开辟了新途径。
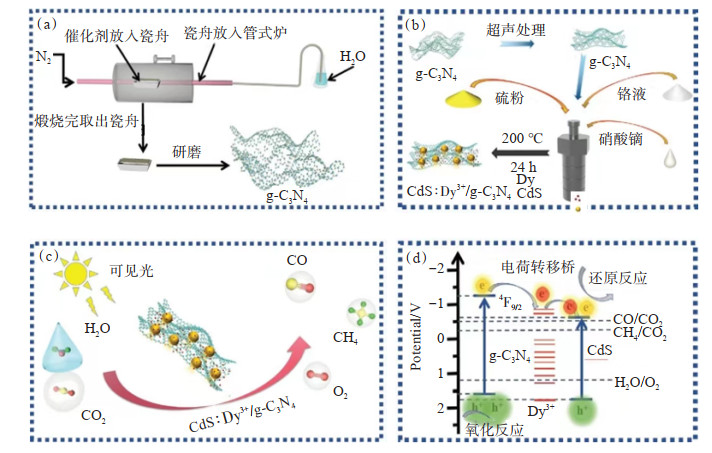
ZHAO等[107]成功构建了g-C3N4和CdS: Dy3+/g-C3N4单原子复合光催化剂(图 2(a)和图 2(b))。实验结果表明,CdS: Dy3+/g-C3N4的催化性能是纯g-C3N4的6.9倍,CdS: Dy3+和CdS: Dy3+/g-C3N4的催化性能分别是纯CdS的7倍和13.7倍。作者描述了CO2还原的过程(图 2(c)),并提出了该催化反应的机理(图 2(d)),证明了稀土单原子Dy3+具有CO2分子活化和4f能级作为电子传递桥的双重作用。同时,说明不是所有的稀土离子都适合于电荷转移桥,这不仅与稀土离子的4f能级有关,还与催化剂的能带结构有关。
![]() 图 2 CdS:Dy3+/CN异质结的合成及机理[107]:(a)g-C3N4的合成;(b)CdS:Dy3+/CN Ⅱ型异质结的合成(c)可见光照射下CO2的光还原过程;(d)光催化CO2还原机理Figure 2. Synthesis and mechanism of CdS: Dy3+/CN heterojunction[107]: (a) Synthesis of g-C3N4; (b) Synthesis of CdS: Dy3+/CN Ⅱ heterojunction; (c) Photoreduction process of CO2 under visible light irradiation; (d) Mechanism of photocatalytic CO2 reduction
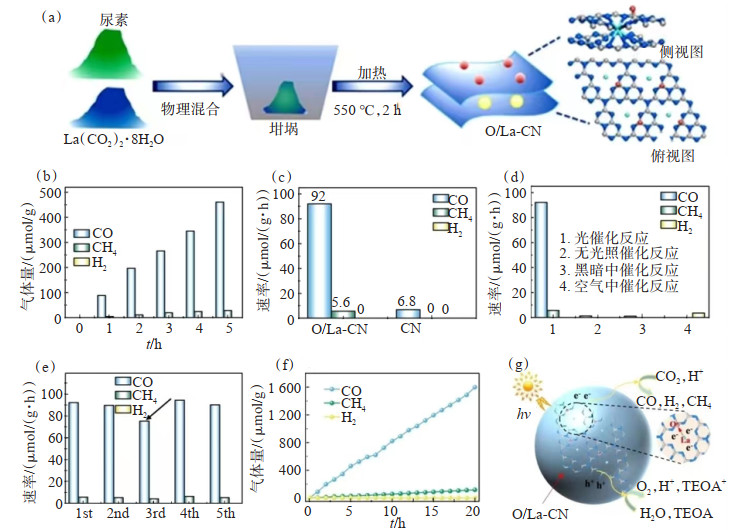
图 2 CdS:Dy3+/CN异质结的合成及机理[107]:(a)g-C3N4的合成;(b)CdS:Dy3+/CN Ⅱ型异质结的合成(c)可见光照射下CO2的光还原过程;(d)光催化CO2还原机理Figure 2. Synthesis and mechanism of CdS: Dy3+/CN heterojunction[107]: (a) Synthesis of g-C3N4; (b) Synthesis of CdS: Dy3+/CN Ⅱ heterojunction; (c) Photoreduction process of CO2 under visible light irradiation; (d) Mechanism of photocatalytic CO2 reductionCHEN等[65]制备了O/La-CN光催化剂(图 3(a)),并在模拟太阳光下进行了光催化CO2还原反应。该系统中检测到的主要产物为CO、H2和CH4。在纯CO2气氛下进行光照,随着时间的推移,O/La-CN表现出显著上升的CO和CH4生成量和还原速率(图 3(b)和图 3(c)),并且CO的选择性高达80.3%。通过控制变量和循环实验证明了O/La-CN是光催化反应以及在长时间反应过程的稳定性(图 3(d),图 3(e),图 3(f))。作者通过该系统的光催化CO2还原机理(图 3(g)清楚地阐明了La单原子4f和5d轨道以及La-N的d轨道杂交原子使电荷转移通道得以形成,表明该催化剂以La-N电荷转移桥作为光催化CO2反应的活性中心, 证明La-N电荷桥是CO2活化、快速生成COOH*和CO解吸的关键活性中心。
![]() 图 3 O/La-CN光催化剂的制备、活性测试及机理[65]:(a)催化剂合成过程示意;(b)光催化CO2还原产量;(c)光还原速率;(d)控制条件的多项实验;(e)循环性实验;(f)稳定性实验;(g)此系统中光催化CO2还原机理Figure 3. Preparation, activity test and mechanism of O/La-CN photocatalyst[65]: (a) Schematic diagram of catalyst synthesis processes; (b) Gas amount of photocatalytic CO2 reduction; (c) Photoreduction rate; (d) Multiple experiments of controlling conditions; (e) cycling experiments; (f) Stability experiment; (g) Photocatalytic CO2 reduction mechanism in this system
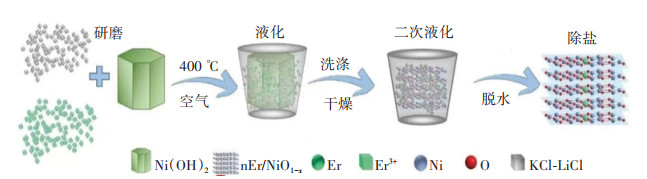
图 3 O/La-CN光催化剂的制备、活性测试及机理[65]:(a)催化剂合成过程示意;(b)光催化CO2还原产量;(c)光还原速率;(d)控制条件的多项实验;(e)循环性实验;(f)稳定性实验;(g)此系统中光催化CO2还原机理Figure 3. Preparation, activity test and mechanism of O/La-CN photocatalyst[65]: (a) Schematic diagram of catalyst synthesis processes; (b) Gas amount of photocatalytic CO2 reduction; (c) Photoreduction rate; (d) Multiple experiments of controlling conditions; (e) cycling experiments; (f) Stability experiment; (g) Photocatalytic CO2 reduction mechanism in this system本研究组通过熔盐法(图 4)加速电荷分离和转移制备了高度分散的Er原子掺杂的含氧空位的n型NiO(Er /NiO1-x)[108]。Er原子的掺杂可以扭曲NiO的晶胞,改变对称性,增强极化和内部电场,有利于光生载流子的有效分离。此外,Er/NiO1-x可以显著提高CO2的吸附和活化,降低CO2光还原反应的能量势垒,使CO2光还原性能优异,较好掺杂比催化剂的CO产率分别是传统NiO和n型NiO的26.3倍和3.9倍。Er/NiO1-x光催化剂和控制内部电场的单胞偶极子为反铁磁材料中的CO2光还原打开了新的窗口。
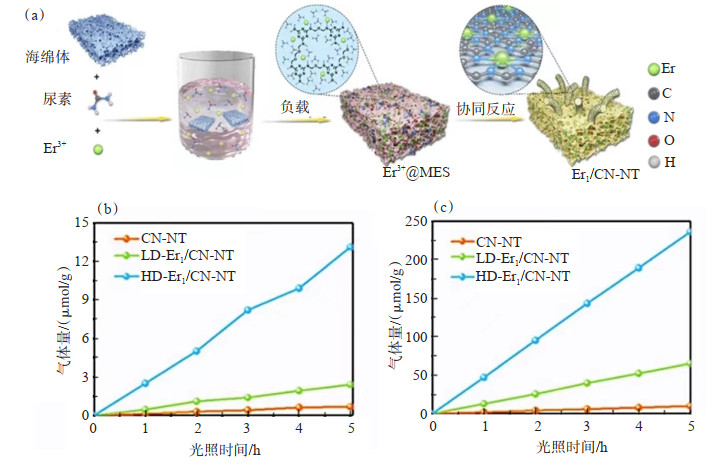
JI等[109]开发了一种原子限制和配位的策略,成功合成了Er原子分散密度可调的稀土单原子Er催化剂(图 5(a))。实验结果和X射线吸收精细结构谱(XAFS)测量以及密度泛函理论(DFT)计算证明了单原子Er位点的形成,表明原子分散的Er原子有助于提高整体光催化CO2还原性能。该研究首次在纯水系统中展示了出色的光催化CO2还原性能。在光照条件下,与低负载单原子Er催化剂(LD-Er1/CN-NT)和CN-NT相比,高负载单原子Er催化剂(HD-Er1/CN-NT)的CH4和CO产率更高,CO和CH4的产率与时间呈良好的线性关系(图 5(b)和图 5(c))。
![]() 图 5 Er1/CN-NT催化剂合成及活性测试[109]:(a)单原子Er1/CN-NT催化剂合成过程示意;(b)采用H2O光催化还原CO2过程中CH4的演化速率;(c)采用H2O光催化还原CO2过程中CO的演化速率Figure 5. Synthesis and activity testing of Er1/CN-NT catalyst[109]: (a) Schematic diagram of the synthesis process of single atom Er1/CN-NT catalyst; (b) Evolution rate of CH4 during the photocatalytic reduction of CO2 using H2O; (c) Evolution rate of CO during the photocatalytic reduction of CO2 using H2O
图 5 Er1/CN-NT催化剂合成及活性测试[109]:(a)单原子Er1/CN-NT催化剂合成过程示意;(b)采用H2O光催化还原CO2过程中CH4的演化速率;(c)采用H2O光催化还原CO2过程中CO的演化速率Figure 5. Synthesis and activity testing of Er1/CN-NT catalyst[109]: (a) Schematic diagram of the synthesis process of single atom Er1/CN-NT catalyst; (b) Evolution rate of CH4 during the photocatalytic reduction of CO2 using H2O; (c) Evolution rate of CO during the photocatalytic reduction of CO2 using H2O3.2 光催化固氮
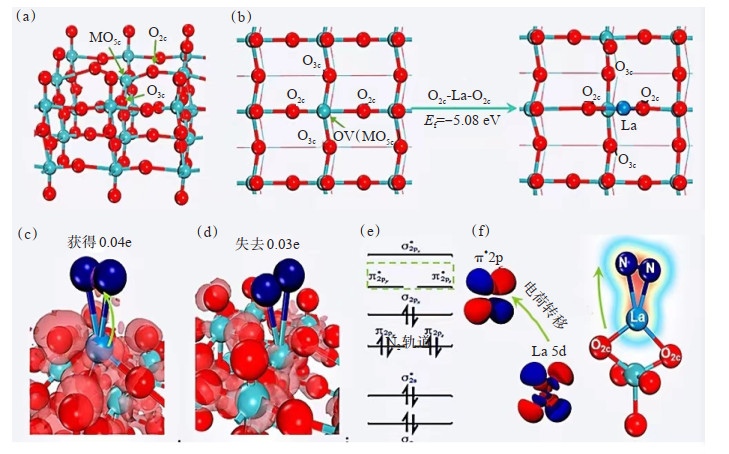
LIU等[98]采用溶剂热-光沉积法制备了La/MoO3-x光催化剂,成功地将单La原子锚定在MoO3-x中,并描述了La/MoO3-x光催化剂的结构(图 6(a)和图 6(b)),对末端氧空位假设构成单La原子锚定位进行了第一性原理计算,以模拟氮在O2c-La-O2c位点上的吸附和活化,从而阐明了La原子的作用。分析电子密度差可知,La/MoO3-x的N-N键中积累了大量电子(图 6(c)),不同于MoO3-x上可忽略的电子积累(图 6(d))。Mulliken电荷分析结果表明氮吸附在La/MoO3-x上可获得0.03个电子,相比吸附在MoO3-x的氮损失0.04个电子。理论上,金属的d轨道与N的2p轨道耦合,促进电子填充到N的π*2p轨道。因此,电子将从单个La原子的5d轨道转移到吸附氮的π*2p轨道(图 6(e)和图 6(f))。DFT结果表明,单个La原子显著优化了局部电子性质,并且吸附在O2c-La-O2c位点上的氮分子可以被激活。该研究还通过HAADF-STEM和XAFS分析证实了单个La原子是以晶格氧配位的方式配位的,并且表明这种具有良好配位结构的单原子La催化剂具有长期稳定性。
![]() 图 6 MoO3-x的结构分析[98]:(a)MoO3-x的优化结构;(b)MoO3-x的俯视结构和La原子在末端氧空位的优化配置;(c)氮和La/MoO3-x间电子密度差;(d)MoO3-x电荷累积的红色等值面;(e)氮的π*2p轨道;(f)从La转移到吸附氮的电子转移图Figure 6. Structural analysis of MoO3-x[98]: (a) Optimization structure of MoO3-x; (b) Top view structure of MoO3-x and optimized configuration of La atoms at terminal oxygen vacancies; (c) Difference in electron density between nitrogen and La/MoO3-x; (d) Red isosurface of charge accumulation in MoO3-x; (e) π*2p orbitals of nitrogen; (f) Electron transfer diagram from La to adsorbed nitrogen
图 6 MoO3-x的结构分析[98]:(a)MoO3-x的优化结构;(b)MoO3-x的俯视结构和La原子在末端氧空位的优化配置;(c)氮和La/MoO3-x间电子密度差;(d)MoO3-x电荷累积的红色等值面;(e)氮的π*2p轨道;(f)从La转移到吸附氮的电子转移图Figure 6. Structural analysis of MoO3-x[98]: (a) Optimization structure of MoO3-x; (b) Top view structure of MoO3-x and optimized configuration of La atoms at terminal oxygen vacancies; (c) Difference in electron density between nitrogen and La/MoO3-x; (d) Red isosurface of charge accumulation in MoO3-x; (e) π*2p orbitals of nitrogen; (f) Electron transfer diagram from La to adsorbed nitrogen3.3 降解有机污染物
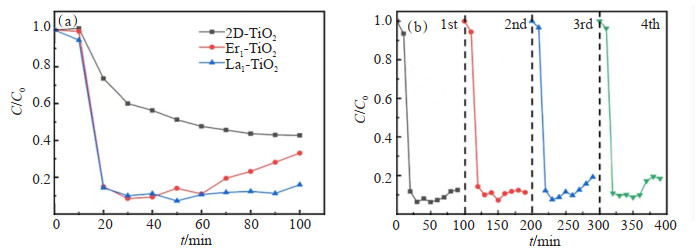
稀土单原子修饰的复合光催化剂在光催化控制空气污染方面具有较大的应用潜力。LI等[86]采用浸渍煅烧法在2D-TiO2纳米片表面修饰了稀土单原子(La,Er),通过AC-HAADF-STEM和X光吸收光谱(XAS)等技术或表征图证实了稀土单原子的形成。实验结果表明,采用稀土单原子(La1-TiO2和Er1-TiO2)修饰的TiO2在邻二甲苯的气相降解方面比纯2D-TiO2表现出更优异的光催化活性(图 7(a)和图 7(b)),作者将其归因于稀土单原子提供的额外的吸附位点降低了邻二甲苯的吸附能,即在不增加比表面积的情况下极大地提高了邻二甲苯的吸附能力。此外,稀土单原子与氧原子结合形成的杂化轨道促进了电荷分离,降低了光生载流子的复合速率,从而提高光催化降解性能。作者利用气相色谱法-质谱法联用(GC-MS)揭示了光催化氧化机理。结果表明,La1-TiO2和Er1-TiO2样品可以减少中间产物的类型并简化反应路线,这表明单原子对中间产物的调节起着重要作用。
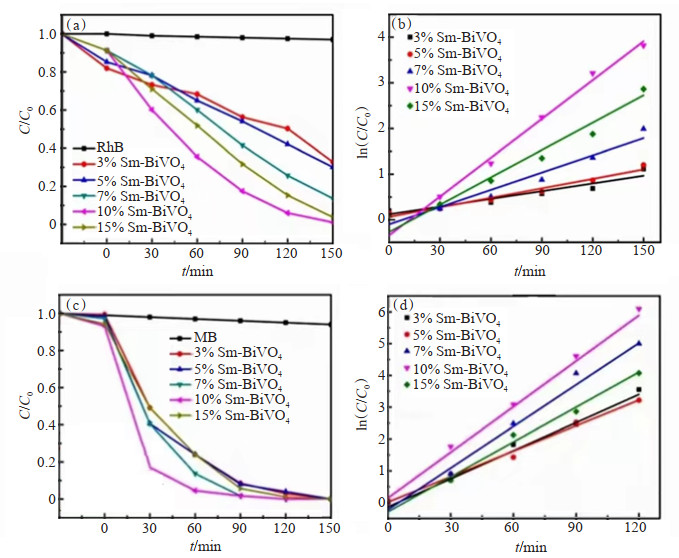
ZHU等[97]采用一锅水热法成功制备了具有混合晶相的BiVO4微球,并以Sm3+调整BiVO4微球形态和光催化活性。SEM结果显示Sm3+修饰的BiVO4为均匀多边形,四方BiVO4的形貌得到了改善。此外,与纯BiVO4相比,Sm3+修饰的BiVO4光催化剂在紫外光和可见光照射(UV-Vis)下能更有效地降解RhB(图 8(a)和图 8(b))和MB(图 8(c)和图 8(d)),作者将其解释为Sm3+通过捕获光诱导电子促进光生电子-空穴对的有效分离,从而提高BiVO4的光催化活性。
![]() 图 8 样品的活性测试[97]:(a)RhB溶液的C/C0与时间的关系;(b)RhB溶液的ln(C/C0)与时间的关系;(c)MB溶液的C/C0与时间的关系;(d)MB溶液的ln(C/C0)与时间的关系Figure 8. Activity testing of samples[97]: (a) Time-course variations of C/C0 of RhB solution; (b) Time-course variations of ln(C/C0) of RhB solution; (c) Time-course variations of C/C0 of MB solution; (d) Time-course variations of ln(C/C0) of MB solution
图 8 样品的活性测试[97]:(a)RhB溶液的C/C0与时间的关系;(b)RhB溶液的ln(C/C0)与时间的关系;(c)MB溶液的C/C0与时间的关系;(d)MB溶液的ln(C/C0)与时间的关系Figure 8. Activity testing of samples[97]: (a) Time-course variations of C/C0 of RhB solution; (b) Time-course variations of ln(C/C0) of RhB solution; (c) Time-course variations of C/C0 of MB solution; (d) Time-course variations of ln(C/C0) of MB solutionSOWIK等[100]采用溶胶-凝胶法制备了Eu、Er、Tb、Yb、Ho、La等修饰的ZnO量子点,增强了其光催化降解活性。实验结果表明,ZnO/RE的结构和光催化性能受稀土金属种类和数量的影响。在ZnO/La和ZnO/Er样品中稀土金属修饰后的ZnO具有比原始量子点更高的发光强度。在所有的ZnO/RE样品中,添加0.45 mmol La的ZnO纳米晶体的发光量子产率最高(约81%)。此外,含有0.09 mmol Er的ZnO/Er样品表现出最佳的光催化性能,即在紫外-可见光辐射下,其对苯酚的去除率达90%,并且与较低的发光强度相一致。ZHANG等[99]通过在沸石型MnO2上分散稀土单原子Y或La,探究了催化剂降解低浓度甲苯的光催化效果。WANG等[92]采用化学沉积法将TiO2沉积在介孔SBA-15分子筛上,并在催化剂表面沉积稀土金属Nd,合成了Nd-TiO2-SBA-15光催化剂。Nd-TiO2-SBA表现出优越的光催化降解甲基橙(MO)偶氮染料的活性。
3.4 光催化有机合成
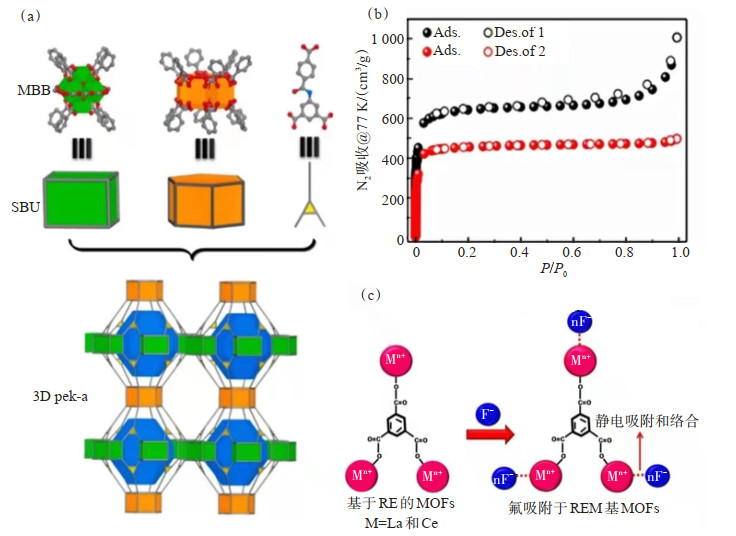
稀土金属有机骨架材料(RE-MOF)因具有独特的发光、磁性和孔隙度等特性而备受关注。WEI等[104]通过拓扑引导法和溶剂热反应法成功设计了2种结构(图 9(a))稀土酰胺功能化MOF。2种MOFs均显示出理想的包含双笼状和通道状孔隙几何形状的拓扑网络。作者通过吸附等温线(图 9(b))确定了2种材料均表现出相对较高的比表面积和孔隙体积。此外,这2种材料在乙烯上选择性吸附丙烯的组成范围均很广,表明其具有广泛的应用前景。
![]() Figure 9. Preparation, characterization testing and mechanism of RE based MOFs photocatalysts: (a) Structure diagram of two compounds; (b) N2 adsorption isotherm of two compounds at 77 K; (c) Plausible fluoride adsorption mechanism of REM based MOFs[105]
Figure 9. Preparation, characterization testing and mechanism of RE based MOFs photocatalysts: (a) Structure diagram of two compounds; (b) N2 adsorption isotherm of two compounds at 77 K; (c) Plausible fluoride adsorption mechanism of REM based MOFs[105]JEYASEELAN等[105]通过热液法成功制备了稀土金属(RE)基MOFs,即La和Ce修饰的苯-1, 3, 5-三羧酸(BTC)。La@BTC和Ce@BTC MOF是最有前途的吸附剂材料,尤其在氟吸附方面。该研究采用多种表征手段对RE基MOF的表面性质和性能进行了表征。考察了初始F-浓度、振荡时间、RE基MOF用量、溶液pH值、温度和干扰阴离子对吸附性能的影响。通过热力学参数、吸附等温线和动力学实验确定了F在REM基MOF上吸附的性质和顺序。研究结果表明,RE基MOF对氟化物的吸附是单层吸附,遵循Langmuir等温线。F在RE基MOF上的吸附所遵循颗粒内扩散和准二级吸附模型,并且该吸附反应是自发的和吸热的。因此,氟化物主要的吸附机理是静电吸引作用和络合作用(图 9(c))。
4 结束语
本文对RE-SAC的合成方法及其在光催化中的应用进行了总结和分析,详细讨论了RE-SAC的各种合成方法及应用,以确定单原子RE活性中心和多相催化机理,并揭示了这些材料的结构-活性关系。将单个稀土原子掺进半导体光催化剂,可以调控光催化剂的电子结构和能带结构,促进催化剂对光的吸收及光生电子和空穴的转移和分离,同时改善光催化剂对反应物吸附和活化的能力;在半导体异质结的基础上引入稀土单原子有望为设计高活性光催化剂开辟新的途径;合成具有大量表面缺陷的半导体,以表面缺陷促进稀土单个原子在其表面的负载和分散,产生强烈的金属-载体相互作用调控光催化剂的表面电子性能和对反应底物的吸附能力提高光催化的活性和稳定性。然而,稀土元素具有独特的物理化学性质,包括化学惰性4f电子、异常的负还原电位、大半径和不饱和配位环境,使研究者很难将过渡金属自组装碳纳米管技术应用于稀土元素。因此,未来需要开发出制备RE-SAC的新有效方法,以期扩大RE-SAC的应用领域。
-
图 1 稀土单原子光催化剂的合成及机理:(a)CdS和CdS/InVO4:Eu3+的合成[96];(b)可见光照射下光催化和光还原CO2过程的机理[96];(c)Eu3+作为ET桥促进电荷转移机理[60];(d)Eu3+作为ET桥促进电荷分离机理[60]
Fig 1. Synthesis and mechanism diagram of rare earth monoatomic photocatalyst: (a) Synthesis diagrams of CdS and CdS/InVO4: Eu3+ [96]; (b) Proposed mechanism diagram of photocatalysis and the photoreduction of CO2 process under visible light irradiation[96]; (c) Mechanism for Eu3+ as ET bridge to promote charge transfer[60]; (d) Mechanism of Eu3+ as ET bridge to promote charge transfer separation[60]
图 2 CdS:Dy3+/CN异质结的合成及机理[107]:(a)g-C3N4的合成;(b)CdS:Dy3+/CN Ⅱ型异质结的合成(c)可见光照射下CO2的光还原过程;(d)光催化CO2还原机理
Fig 2. Synthesis and mechanism of CdS: Dy3+/CN heterojunction[107]: (a) Synthesis of g-C3N4; (b) Synthesis of CdS: Dy3+/CN Ⅱ heterojunction; (c) Photoreduction process of CO2 under visible light irradiation; (d) Mechanism of photocatalytic CO2 reduction
图 3 O/La-CN光催化剂的制备、活性测试及机理[65]:(a)催化剂合成过程示意;(b)光催化CO2还原产量;(c)光还原速率;(d)控制条件的多项实验;(e)循环性实验;(f)稳定性实验;(g)此系统中光催化CO2还原机理
Fig 3. Preparation, activity test and mechanism of O/La-CN photocatalyst[65]: (a) Schematic diagram of catalyst synthesis processes; (b) Gas amount of photocatalytic CO2 reduction; (c) Photoreduction rate; (d) Multiple experiments of controlling conditions; (e) cycling experiments; (f) Stability experiment; (g) Photocatalytic CO2 reduction mechanism in this system
图 5 Er1/CN-NT催化剂合成及活性测试[109]:(a)单原子Er1/CN-NT催化剂合成过程示意;(b)采用H2O光催化还原CO2过程中CH4的演化速率;(c)采用H2O光催化还原CO2过程中CO的演化速率
Fig 5. Synthesis and activity testing of Er1/CN-NT catalyst[109]: (a) Schematic diagram of the synthesis process of single atom Er1/CN-NT catalyst; (b) Evolution rate of CH4 during the photocatalytic reduction of CO2 using H2O; (c) Evolution rate of CO during the photocatalytic reduction of CO2 using H2O
图 6 MoO3-x的结构分析[98]:(a)MoO3-x的优化结构;(b)MoO3-x的俯视结构和La原子在末端氧空位的优化配置;(c)氮和La/MoO3-x间电子密度差;(d)MoO3-x电荷累积的红色等值面;(e)氮的π*2p轨道;(f)从La转移到吸附氮的电子转移图
Fig 6. Structural analysis of MoO3-x[98]: (a) Optimization structure of MoO3-x; (b) Top view structure of MoO3-x and optimized configuration of La atoms at terminal oxygen vacancies; (c) Difference in electron density between nitrogen and La/MoO3-x; (d) Red isosurface of charge accumulation in MoO3-x; (e) π*2p orbitals of nitrogen; (f) Electron transfer diagram from La to adsorbed nitrogen
图 8 样品的活性测试[97]:(a)RhB溶液的C/C0与时间的关系;(b)RhB溶液的ln(C/C0)与时间的关系;(c)MB溶液的C/C0与时间的关系;(d)MB溶液的ln(C/C0)与时间的关系
Fig 8. Activity testing of samples[97]: (a) Time-course variations of C/C0 of RhB solution; (b) Time-course variations of ln(C/C0) of RhB solution; (c) Time-course variations of C/C0 of MB solution; (d) Time-course variations of ln(C/C0) of MB solution
图 9 RE基MOFs光催化剂的制备、表征测试及机理:(a)两种化合物的结构示意[104];(b)两种化合物在77 K下的N2吸附等温线[104];(c)RE基MOFs对氟化物的吸附机理[105]
Fig 9. Preparation, characterization testing and mechanism of RE based MOFs photocatalysts: (a) Structure diagram of two compounds; (b) N2 adsorption isotherm of two compounds at 77 K; (c) Plausible fluoride adsorption mechanism of REM based MOFs[105]
-
[1] 张萌迪, 陈范云, 马小帅, 等. 纳微结构Ag2CO3光催化材料的制备及其在光催化的应用[J]. 有色金属科学与工程, 2019, 10(2): 52-61. doi: 10.13264/j.cnki.ysjskx.2019.02.008 [2] 白羽, 吴榛, 刘仁月, 等. 花状Pt/Bi2WO6微米晶合成、表征及其高可见光催化性能[J]. 有色金属科学与工程, 2016, 7(2): 60-66. doi: 10.13264/j.cnki.ysjskx.2016.02.011 [3] 张川群, 周勤, 徐冲, 等. Bi2MoO6的形貌调控及其应用研究进展[J]. 有色金属科学与工程, 2021, 12(2): 56-65. doi: 10.13264/j.cnki.ysjskx.2021.02.008 [4] 王颖, 杨传玺, 王小宁, 等. 二维光催化材料研究进展[J]. 有色金属科学与工程, 2021, 12(2): 30-42. doi: 10.13264/j.cnki.ysjskx.2021.02.005 [5] 杜瑞安, 马小帅, 张萌迪, 等. 多壁碳纳米管/TiO2复合材料的合成及其光催化性能[J]. 有色金属科学与工程, 2019, 10(5): 75-84. doi: 10.13264/j.cnki.ysjskx.2019.05.012 [6] DRESSELHAUS M S, THOMAS I L. Alternative energy technologies[J]. Nature, 2001, 414(6861): 332-337. doi: 10.1038/35104599
[7] LEWIS N S, NOCERA D G. Powering the planet: chemical challenges in solar energy utilization[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(43): 15729-15735. doi: 10.1073/pnas.0603395103
[8] GAO C, LOW J, LONG R, et al. Heterogeneous single-atom photocatalysts: fundamentals and applications[J]. Chemical Reviews, 2020, 120(21): 12175-12216. doi: 10.1021/acs.chemrev.9b00840
[9] TACHIBANA Y, VAYSSIERES L, DURRANT J R. Artificial photosynthesis for solar water-splitting[J]. Nature Photonics, 2012, 6(8): 511-518. doi: 10.1038/nphoton.2012.175
[10] LISTORTI A, DURRANT J, BARBER J. Solar to fuel[J]. Nature Materials, 2009, 8(12): 929-930. doi: 10.1038/nmat2578
[11] LI Y R, KONG T T, SHEN S H. Artificial photosynthesis with polymeric carbon nitride: when meeting metal nanoparticles, single atoms, and molecular complexes[J]. Small (Weinheim an Der Bergstrasse, Germany), 2019, 15(32): e1900772. doi: 10.1002/smll.201900772
[12] KOU J H, LU C H, WANG J, et al. Selectivity enhancement in heterogeneous photocatalytic transformations[J]. Chemical Reviews, 2017, 117(3): 1445-1514. doi: 10.1021/acs.chemrev.6b00396
[13] WENDERICH K, MUL G. Methods, mechanism, and applications of photodeposition in photocatalysis: a review[J]. Chemical Reviews, 2016, 116(23): 14587-14619. doi: 10.1021/acs.chemrev.6b00327
[14] GAO C, WANG J, XU H X, et al. Coordination chemistry in the design of heterogeneous photocatalysts[J]. Chemical Society Reviews, 2017, 46(10): 2799-2823. doi: 10.1039/C6CS00727A
[15] LINSEBIGLER A L, LU G Q, YATES J T Jr. Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results[J]. Chemical Reviews, 1995, 95(3): 735-758. doi: 10.1021/cr00035a013
[16] SCHNEIDER J, MATSUOKA M, TAKEUCHI M, et al. Understanding TiO2 photocatalysis: mechanisms and materials[J]. Chemical Reviews, 2014, 114(19): 9919-9986. doi: 10.1021/cr5001892
[17] HE Y C, SHEN L S, XIE Z J, et al. Submicrostructure and typing of female genital condylomata[J]. Chinese Medical Journal, 1993, 106(4): 298-302.
[18] LU H J, TOURNET J, DASTAFKAN K, et al. Noble-metal-free multicomponent nanointegration for sustainable energy conversion[J]. Chemical Reviews, 2021, 121(17): 10271-10366. doi: 10.1021/acs.chemrev.0c01328
[19] DUPRÉ L. Marx's critique of culture and its interpretations[J]. The Review of Metaphysics, 1980, 34(1): 91-121.
[20] QIAO B T, WANG A Q, YANG X F, et al. Single-atom catalysis of CO oxidation using Pt1/FeOx[J]. Nature Chemistry, 2011, 3(8): 634-641. doi: 10.1038/nchem.1095
[21] XU H, ZHANG Z, LIU J, et al. Entropy-stabilized single-atom Pd catalysts via high-entropy fluorite oxide supports[J]. Nature Communications, 2020, 11(1): 3908. U. doi: 10.1038/s41467-020-17738-9
[22] WANG A Q, LI J, ZHANG T. Heterogeneous single-atom catalysis[J]. Nature Reviews Chemistry, 2018, 2(6): 65-81. doi: 10.1038/s41570-018-0010-1
[23] LIU J, JIAO M G, MEI B B, et al. Carbon-supported divacancy-anchored platinum single-atom electrocatalysts with superhigh Pt utilization for the oxygen reduction reaction[J]. Angewandte Chemie International Edition, 2019, 58(4): 1163-1167. doi: 10.1002/anie.201812423
[24] ZHANG H B, LIU G G, SHI L, et al. Single-atom catalysts: emerging multifunctional materials in heterogeneous catalysis[J]. Advanced Energy Materials, 2018, 8(1): 1701343. doi: 10.1002/aenm.201701343
[25] ZHANG B, FAN T J, XIE N, et al. Versatile applications of metal single-atom @ 2D material nanoplatforms[J]. Advanced Science, 2019, 6(21): 1901787. doi: 10.1002/advs.201901787
[26] JIANG K, SIAHROSTAMI S, ZHENG T T, et al. Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction[J]. Energy & Environmental Science, 2018, 11(4): 893-903.
[27] HU Y S, LEE C C, GUO W Y, et al. Trigeminal nerve atrophy predicts pain recurrence after gamma knife stereotactic radiosurgery for classical trigeminal neuralgia[J]. Neurosurgery, 2019, 84(4): 927-934. doi: 10.1093/neuros/nyy122
[28] CHEN Z L, GU H Y, LI Y, et al. Safety assessment for genetically modified sweet pepper and tomato[J]. Toxicology, 2003, 188(2/3): 297-307.
[29] SONG L, MA W Z, YAO Y L, et al. Bole bryophyte diversity and distribution patterns along three altitudinal gradients in Yunnan, China[J]. Journal of Vegetation Science, 2015, 26(3): 576-587. doi: 10.1111/jvs.12263
[30] LIU S H, YANG B J, GU J H, et al. Molecular cloning and characterization of a juvenile hormone esterase gene from brown planthopper, Nilaparvata lugens[J]. Journal of Insect Physiology, 2008, 54(12): 1495-1502. doi: 10.1016/j.jinsphys.2008.08.010
[31] SUN G X, YANG M Y, SUN Z H, et al. Synthesis and bioactivities of novel 1, 3, 4-oxadiazole derivatives containing 1, 2, 3-thiadiazole moiety[J]. Phosphorus, Sulfur, and Silicon and the Related Elements, 2014, 189(12): 1895-1900. doi: 10.1080/10426507.2014.906424
[32] SLAVINSKAYA D E M, STADNICHENKO D A I, MURAVYOV V V, et al. Transformation of a Pt-CeO2 mechanical mixture of pulsed‐laser‐ablated nanoparticles to a highly active catalyst for carbon monoxide oxidation[J]. Chem Cat Chem, 2018, 10(10): 2232-2247. doi: 10.1002/cctc.201702050/asset/supinfo/cctc201702050-sup-0001-misc_information.pdf?v=1&s=3ecb20c42bd690a6bdb99c72bb5f673e41c62cb1
[33] XU X J, FANG S, LI Y H, et al. Effects of low acyl and high acyl gellan gum on the thermal stability of purple sweet potato anthocyanins in the presence of ascorbic acid[J]. Food Hydrocolloids, 2019, 86: 116-123. doi: 10.1016/j.foodhyd.2018.03.007
[34] WANG H Y, LIU X Y, GUO L, et al. Effects of MISA A on actin cytoskeleton of cultured HTM cells and intraocular pressure of rats and glaucomatous monkeys[J]. Current Eye Research, 2007, 32(10): 843-850. doi: 10.1080/02713680701585880
[35] CHOI J, YU T, CHA B C, et al. Modulatory effects of ZYM-201 sodium succinate on dietary-induced hyperlipidemic conditions[J]. Die Pharmazie, 2011, 66(10): 791-797.
[36] LIU C J, CHENG D G, ZHANG Y P, et al. Remarkable enhancement in the dispersion and low-temperature activity of catalysts prepared via novel plasma reduction-calcination method[J]. Catalysis Surveys from Asia, 2004, 8(2): 111-118. doi: 10.1023/B:CATS.0000026991.40338.c9
[37] FENG Y X, WAN Q, XIONG H F, et al. Correlating DFT calculations with CO oxidation reactivity on Ga-doped Pt/CeO2 single-atom catalysts[J]. The Journal of Physical Chemistry C, 2018, 122(39): 22460-22468. doi: 10.1021/acs.jpcc.8b05815
[38] QI J M, GAO L Y, WEI F F, et al. Design of a high-performance electrocatalyst for N2 conversion to NH3 by trapping single metal atoms on stepped CeO2[J]. ACS Applied Materials & Interfaces, 2019, 11(50): 47525-47534.
[39] HU N, XIE W Q, LIU J R, et al. Modeling of GaAs multiplier Schottky diode in terahertz band[C]//(Shanghai): 2019 IEEE International Conference on Computational Electromagnetics (ICCEM), 2019, 1-3.
[40] XU H D, ZHANG Z H, LIU J X, et al. Entropy-stabilized single-atom Pd catalysts via high-entropy fluorite oxide supports[J]. Nature Communications, 2020, 11(1): 1-9. doi: 10.1038/s41467-019-13993-7
[41] XU D W, HE Y B, CHU X D, et al. Synthesis of lithium iron phosphate/carbon microspheres by using polyacrylic acid coated iron phosphate nanoparticles derived from iron(Ⅲ) acrylate[J]. Chem Sus Chem, 2015, 8(6): 1009-1016. doi: 10.1002/cssc.201403060
[42] WANG B, CAI H R, SHEN S H. Single metal atom photocatalysis[J]. Small Methods, 2019, 3(9): 1800447. doi: 10.1002/smtd.201800447
[43] LI Z, JI S F, LIU Y W, et al. Well-defined materials for heterogeneous catalysis: from nanoparticles to isolated single-atom sites[J]. Chemical Reviews, 2020, 120(2): 623-682. doi: 10.1021/acs.chemrev.9b00311
[44] LIU K P, TANG Y, YU Z Y, et al. High-loading and thermally stable Pt1/MgAl1.2Fe0.8O4 single-atom catalysts for high-temperature applications[J]. Science China Materials, 2020, 63(6): 949-958. doi: 10.1007/s40843-020-1267-2
[45] NGUYEN C H, FU C C, JUANG R S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: efficiency and degradation pathways[J]. Journal of Cleaner Production, 2018, 202: 413-427. doi: 10.1016/j.jclepro.2018.08.110
[46] LIU J Y, KONG X, ZHENG L R, et al. Rare earth single-atom catalysts for nitrogen and carbon dioxide reduction[J]. ACS Nano, 2020, 14(1): 1093-1101. doi: 10.1021/acsnano.9b08835
[47] WANG Q S, ZHANG D F, CHEN Y, et al. Single-atom catalysts for photocatalytic reactions[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(7): 6430-6443.
[48] CHEN Y J, JI S F, SUN W M, et al. Engineering the atomic interface with single platinum atoms for enhanced photocatalytic hydrogen production[J]. Angewandte Chemie International Edition, 2020, 59(3): 1295-1301. doi: 10.1002/anie.201912439
[49] QIU S J, SHEN Y L, WEI G J, et al. Carbon dots decorated ultrathin CdS nanosheets enabling in-situ anchored Pt single atoms: a highly efficient solar-driven photocatalyst for hydrogen evolution[J]. Applied Catalysis B: Environmental, 2019, 259: 118036. doi: 10.1016/j.apcatb.2019.118036
[50] ZENG L, DAI C H, LIU B, et al. Oxygen-assisted stabilization of single-atom Au during photocatalytic hydrogen evolution[J]. Journal of Materials Chemistry A, 2019, 7(42): 24217-24221. doi: 10.1039/C9TA10290F
[51] MASSARI S, RUBERTI M. Rare earth elements as critical raw materials: focus on international markets and future strategies[J]. Resources Policy, 2013, 38(1): 36-43. doi: 10.1016/j.resourpol.2012.07.001
[52] BOSKOVIC C. Rare earth polyoxometalates[J]. Accounts of Chemical Research, 2017, 50(9): 2205-2214. doi: 10.1021/acs.accounts.7b00197
[53] ZHAO Z W, QIN J, ZHANG C, et al. Recyclable single-component rare-earth metal catalysts for cycloaddition of CO2 and epoxides at atmospheric pressure[J]. Inorganic Chemistry, 2017, 56(8): 4569-4576.
[54] MIGASZEWSKI Z M, GAŁUSZKA A. The characteristics, occurrence, and geochemical behavior of rare earth elements in the environment: a review[J]. Critical Reviews in Environmental Science and Technology, 2015, 45(5): 429-471. doi: 10.1080/10643389.2013.866622
[55] BINNEMANS K, JONES P T, BLANPAIN B, et al. Recycling of rare earths: a critical review[J]. Journal of Cleaner Production, 2013, 51: 1-22. doi: 10.1016/j.jclepro.2012.12.037
[56] BÜNZLI J C G, ELISEEVA S V. Photophysics of lanthanoid coordination compounds[C]//Amsterdam: Elsevier, Comprehensive Inorganic Chemistry Ⅱ, 2013: 339-398.
[57] KAISER S K, CHEN Z P, FAUST AKL D, et al. Single-atom catalysts across the periodic table[J]. Chemical Reviews, 2020, 120(21): 11703-11809. doi: 10.1021/acs.chemrev.0c00576
[58] MING Z, WEN X U, LI W U, et al. Recent progress in MXene-based nanomaterials for high-performance aqueous zinc-ion hybrid capacitors[J]. New Carbon Materials, 2022, 37(3): 508-526. doi: 10.1016/S1872-5805(22)60611-5
[59] WANG X, ZHU Y, LI H, et al. Rare-earth single-atom catalysts: a new frontier in photo/electrocatalysis[J]. Small Methods, 2022, 6(8): e2200413. doi: 10.1002/smtd.202200413
[60] CHEN Q, GAO G, ZHANG Y, et al. Dual functions of CO2 molecular activation and 4f levels as electron transport bridges in erbium single atom composite photocatalysts therefore enhancing visible-light photoactivities[J]. Journal of Materials Chemistry A, 2021, 9(28): 15820-15826. doi: 10.1039/D1TA02926F
[61] WANG S W, BORISEVICH A Y, RASHKEEV S N, et al. Dopants adsorbed as single atoms prevent degradation of catalysts[J]. Nature Materials, 2004, 3(3): 143-146. doi: 10.1038/nmat1077
[62] NGUYEN T N, EBRAHIM F M, STYLIANOU K C. Photoluminescent, upconversion luminescent and nonlinear optical met al-organic frameworks: from fundamental photophysics to potential applications[J]. Coordination Chemistry Reviews, 2018, 377: 259-306. doi: 10.1016/j.ccr.2018.08.024
[63] KARMAKAR A, SAMANTA P, DUTTA S, et al. Front cover: fluorescent "turn-on" sensing based on metal-organic frameworks (MOFs)[J]. Chemistry-An Asian Journal, 2019, 14(24): 4430. doi: 10.1002/asia.201901457
[64] CHEN F, WANG Y M, GUO W W, et al. Color-tunable lanthanide metal-organic framework gels[J]. Chemical Science, 2018, 10(6): 1644-1650.
[65] CHEN P, LEI B, DONG X A, et al. Rare-earth single-atom La-N charge-transfer bridge on carbon nitride for highly efficient and selective photocatalytic CO2 reduction[J]. ACS Nano, 2020, 14(11): 15841-15852. doi: 10.1021/acsnano.0c07083
[66] DU J M, ZHAO G Y, PANG H, et al. A template method for synthesis of porous Sn-doped TiO2 monolith and its enhanced photocatalytic activity[J]. Materials Letters, 2013, 93: 419-422. doi: 10.1016/j.matlet.2012.11.134
[67] TANG T, YIN Z L, CHEN J R, et al. Novel p-n heterojunction Bi2O3/Ti3+-TiO2 photocatalyst enables the complete removal of tetracyclines under visible light[J]. Chemical Engineering Journal, 2021, 417: 128058. doi: 10.1016/j.cej.2020.128058
[68] ZENG Z C, XU Y S, ZHANG Z S, et al. Rare-earth-containing perovskite nanomaterials: design, synthesis, properties and applications[J]. Chemical Society Reviews, 2020, 49(4): 1109-1143. doi: 10.1039/C9CS00330D
[69] YAN X H, FANG M F, QIAN Z Q, et al. Isolation and characterization of polymorphic microsatellites in the perennial herb Euphorbia kansui using paired-end illumina shotgun sequencing[J]. Conservation Genetics Resources, 2014, 6(4): 841-843. doi: 10.1007/s12686-014-0274-9
[70] SUN J F, XU Q Q, QI J L, et al. Isolated single atoms anchored on N-doped carbon materials as a highly efficient catalyst for electrochemical and organic reactions[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(39): 14630-14656.
[71] ZHANG Q Z, HE J, YU W M, et al. A promising anticancer drug: a photosensitizer based on the porphyrin skeleton[J]. RSC Medicinal Chemistry, 2020, 11(4): 427-437. doi: 10.1039/C9MD00558G
[72] NIU J Y, CHEN G, ZHAO J W, et al. Two novel copper-undecaniobates decorated by copper-organic cations [{Cu(H2O)L}2{CuNb11O35H4}]5- (L=1, 10-phenanthroline, 2, 2'-bipyridine) consisting of plenary and monolacunary Lindqvist-type isopolyniobate fragments[J]. Chemistry (Weinheim an Der Bergstrasse, Germany), 2010, 16(24): 7082-7086.
[73] WAN Y S, LIU D Y, WANG S J, et al. ∼2.7Ga juvenile crust formation in the North China Craton (Taishan-Xintai area, western Shandong Province): further evidence of an understated event from U-Pb dating and Hf isotopic composition of zircon[J]. Precambrian Research, 2011, 186(1-4): 169-180. doi: 10.1016/j.precamres.2011.01.015
[74] ZHANG R, XU J, LU C H, et al. Photothermal application of SmCoO3/SBA-15 catalysts synthesized by impregnation method[J]. Materials Letters, 2018, 228: 199-202. doi: 10.1016/j.matlet.2018.05.129
[75] PARNICKA P, MAZIERSKI P, LISOWSKI W, et al. A new simple approach to prepare rare-earth met als-modified TiO2 nanotube arrays photoactive under visible light: surface properties and mechanism investigation[J]. Results in Physics, 2019, 12: 412-423. doi: 10.1016/j.rinp.2018.11.073
[76] BANDI V R, RAGHAVAN C M, GRANDHE B K, et al. Synthesis, structural and optical properties of pure and rare-earth ion doped TiO2 nanowire arrays by a facile hydrothermal technique[J]. Thin Solid Films, 2013, 547: 207-211. doi: 10.1016/j.tsf.2013.03.039
[77] WANG Y, ARANDIYAN H, SCOTT J, et al. Single atom and nanoclustered Pt catalysts for selective CO2 reduction[J]. ACS Applied Energy Materials, 2018, 1(12): 6781-6789. doi: 10.1021/acsaem.8b00817
[78] PARNICKA P, MAZIERSKI P, GRZYB T, et al. Preparation and photocatalytic activity of Nd-modified TiO2 photocatalysts: Insight into the excitation mechanism under visible light[J]. Journal of Catalysis, 2017, 353: 211-222. doi: 10.1016/j.jcat.2017.07.017
[79] NWE T S, SIKONG L, KOKOO R, et al. Photocatalytic activity enhancement of Dy-doped TiO2 nanoparticles hybrid with TiO2(B)nanobelts under UV and fluorescence irradiation[J]. Current Applied Physics, 2020, 20(2): 249-254. doi: 10.1016/j.cap.2019.11.008
[80] STEIN J, de la TORRE-UBIETA L, TIAN Y, et al. A quantitative framework to evaluate modeling of cortical development by neural stem cells[J]. Neuron, 2014, 83(1): 69-86. doi: 10.1016/j.neuron.2014.05.035
[81] GAN T, HE Q, ZHANG H, et al. Unveiling the kilogram-scale gold single-atom catalysts via ball milling for preferential oxidation of CO in excess hydrogen[J]. Chemical Engineering Journal, 2020, 389: 124490. doi: 10.1016/j.cej.2020.124490
[82] LIU Z Q, LI N, ZHAO H Y, et al. Regulating the active species of Ni(OH)2 using CeO2: 3D CeO2/Ni(OH)2/carbon foam as an efficient electrode for the oxygen evolution reaction[J]. Chemical Science, 2017, 8(4): 3211-3217. doi: 10.1039/C6SC05408K
[83] JIANG D, WAN G, GARCÍA-VARGAS C E, et al. Elucidation of the active sites in single-atom Pd1/CeO2 catalysts for low-temperature CO oxidation[J]. ACS Catalysis, 2020, 10(19): 11356-11364. doi: 10.1021/acscatal.0c02480
[84] LI J C, QIN X P, XIAO F, et al. Highly dispersive cerium atoms on carbon nanowires as oxygen reduction reaction electrocatalysts for Zn-air batteries[J]. Nano Letters, 2021, 21(10): 4508-4515. doi: 10.1021/acs.nanolett.1c01493
[85] LI J C, MAURYA S, KIM Y S, et al. Stabilizing single-atom iron electrocatalysts for oxygen reduction via ceria confining and trapping[J]. ACS Catalysis, 2020, 10(4): 2452-2458. doi: 10.1021/acscatal.9b04621
[86] LI J, CHEN S, QUAN F J, et al. Accelerated dinitrogen electroreduction to ammonia via interfacial polarization triggered by single-atom protrusions[J]. Chem., 2020, 6(4): 885-901. doi: 10.1016/j.chempr.2020.01.013
[87] DEREVYANNIKOVA E, KARDASH T Y, STADNICHENKO A I, et al. Structural insight into strong Pt-CeO2 interaction: from single Pt atoms to PtOx clusters[J]. The Journal of Physical Chemistry C, 2019, 123(2): 1320-1334. doi: 10.1021/acs.jpcc.8b11009
[88] ZHU M Z, ZHAO C, LIU X K, et al. Single atomic cerium sites with a high coordination number for efficient oxygen reduction in proton-exchange membrane fuel cells[J]. ACS Catalysis, 2021, 11(7): 3923-3929. doi: 10.1021/acscatal.0c05503
[89] HAZRA D, TSAVDARIS N, MUKHTAROVA A, et al. Superconducting properties of NbTiN thin films deposited by high-temperature chemical vapor deposition[J]. Physical Review B, 2018, 97(14): 144518. doi: 10.1103/PhysRevB.97.144518
[90] YE X X, WANG H W, LIN Y, et al. Insight of the stability and activity of platinum single atoms on ceria[J]. Nano Research, 2019, 12(6): 1401-1409. doi: 10.1007/s12274-019-2351-6
[91] WANG S, WANG Z Y, WANG Y, et al. Study on the controlled synthesis and photocatalytic performance of rare earth Nd deposited on mesoporous TiO2 photocatalysts[J]. Science of the Total Environment, 2019, 652: 85-92. doi: 10.1016/j.scitotenv.2018.10.154
[92] WANG Y F, CHEN Z, HAN P, et al. Single-atomic Cu with multiple oxygen vacancies on ceria for electrocatalytic CO2 reduction to CH4[J]. ACS Catalysis, 2018, 8(8): 7113-7119. doi: 10.1021/acscatal.8b01014
[93] WADA S, OKA K, WATANABE K, et al. Catalytic conversion of carbon dioxide into dimethyl carbonate using reduced copper-cerium oxide catalysts as low as 353 K and 1.3 MPa and the reaction mechanism[J]. Frontiers in Chemistry, 2013, 1: 8.
[94] 刘小华, 孙荣林. 水热与溶剂热合成技术在无机合成中的应用[J]. 盐湖研究, 2008, 16(2): 60-65. https://www.cnki.com.cn/Article/CJFDTOTAL-YHYJ200802016.htm [95] KASUGA T, HIRAMATSU M, HOSON A, et al. Formation of titanium oxide nanotube[J]. Langmuir, 1998, 14(12): 3160-3163. doi: 10.1021/la9713816
[96] LI Y N, QU Y, WANG G F. Europium single atom based heterojunction photocatalysts with enhanced visible-light catalytic activity[J]. Journal of Materials Chemistry A, 2022, 10(11): 5990-5997. doi: 10.1039/D1TA08253A
[97] ZHU S W, LI Q G, HUTTULA M, et al. One-pot hydrothermal synthesis of BiVO4 microspheres with mixed crystal phase and Sm3+-doped BiVO4 for enhanced photocatalytic activity[J]. Journal of Materials Science, 2017, 52(3): 1679-1693. doi: 10.1007/s10853-016-0460-0
[98] LIU Q T, LI Y C, ZHENG L R, et al. Sequential synthesis and active-site coordination principle of precious metal single-atom catalysts for oxygen reduction reaction and PEM fuel cells[J]. Advanced Energy Materials, 2020, 10(20): 2000689. doi: 10.1002/aenm.202000689
[99] ZHANG H Y, ZHENG X M, XU T Z, et al. Atomically dispersed Y or La on birnessite-type MnO2 for the catalytic decomposition of low-concentration toluene at room temperature[J]. ACS Applied Materials & Interfaces, 2021, 13(15): 17532-17542.
[100] SOWIK J, MIODYŃSKA M, BAJOROWICZ B, et al. Optical and photocatalytic properties of rare earth metal-modified ZnO quantum dots[J]. Applied Surface Science, 2019, 464: 651-663. doi: 10.1016/j.apsusc.2018.09.104
[101] WANG C X, LUO G, NISHIURA M, et al. Heteroatom-assisted olefin polymerization by rare-earth metal catalysts[J]. Science Advances, 2017, 3(7): e1701011. doi: 10.1126/sciadv.1701011
[102] HU F Z, LIAO L L, CHI B Z, et al. Rare earth praseodymium-based single atom catalyst for high performance CO2 reduction reaction[J]. Chemical Engineering Journal, 2022, 436: 135271. doi: 10.1016/j.cej.2022.135271
[103] WU Z B, DONG F, ZHAO W R, et al. The fabrication and characterization of novel carbon doped TiO2 nanotubes, nanowires and nanorods with high visible light photocatalytic activity[J]. Nanotechnology, 2009, 20(23): 235701. doi: 10.1088/0957-4484/20/23/235701
[104] WEI W Q, GUO X N, ZHANG Z H, et al. Topology-guided synthesis and construction of amide-functionalized rare-earth metal-organic frameworks[J]. Inorganic Chemistry Communications, 2021, 133: 108896. doi: 10.1016/j.inoche.2021.108896
[105] JEYASEELAN A, VISWANATHAN N. Facile synthesis of tunable rare earth based metal organic frameworks for enhanced fluoride retention[J]. Journal of Molecular Liquids, 2021, 326: 115163. doi: 10.1016/j.molliq.2020.115163
[106] HAN Z D, ZHAO Y, GAO G Y, et al. Erbium single atom composite photocatalysts for reduction of CO2 under visible light: CO2 molecular activation and 4f levels as an electron transport bridge[J]. Small, 2021, 17(26): 2102089. doi: 10.1002/smll.202102089
[107] ZHAO D M, DONG C L, WANG B, et al. Synergy of dopants and defects in graphitic carbon nitride with exceptionally modulated band structures for efficient photocatalytic oxygen evolution[J]. Advanced Materials, 2019, 31(43): 1903545. doi: 10.1002/adma.201903545
[108] YU Z Z, YANG K, YU C L, et al. Steering unit cell dipole and internal electric field by highly dispersed Er atoms embedded into NiO for efficient CO2 photoreduction[J]. Advanced Functional Materials, 2022, 32(28): 2111999. doi: 10.1002/adfm.202111999
[109] JI S F, QU Y, WANG T, et al. Rare-earth single erbium atoms for enhanced photocatalytic CO2 reduction[J]. Angewandte Chemie International Edition, 2020, 59(26): 10651-10657. doi: 10.1002/anie.202003623
-
期刊类型引用(2)
1. 冯强,李健. 铜基催化剂电催化CO_2还原研究进展. 有色金属科学与工程. 2024(03): 364-382 .  本站查看
本站查看
2. 王书红,陈步东,曹飞飞,吴启军,钱纯波,徐飞星,李中平. TiO_2/CuS复合材料的制备及其光催化降解污染废水的性能研究. 有色金属科学与工程. 2024(06): 877-889 .  本站查看
本站查看
其他类型引用(2)



 下载:
下载: