Effect of rare earth Yttrium on the tensile properties of 6.5%Si steel
-
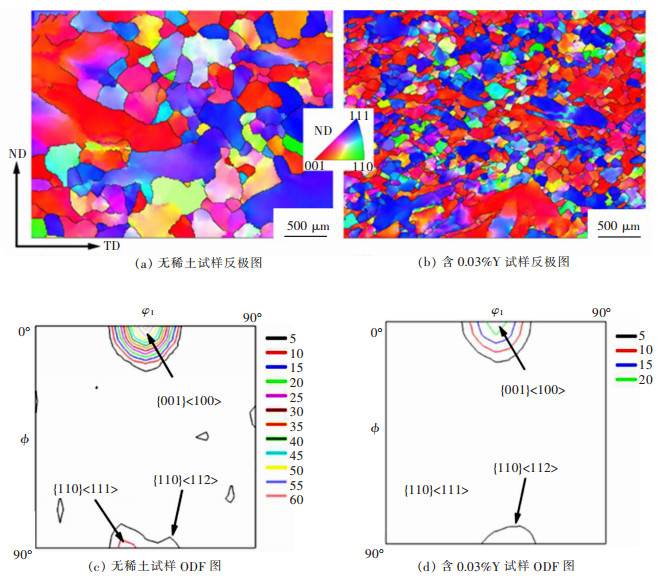
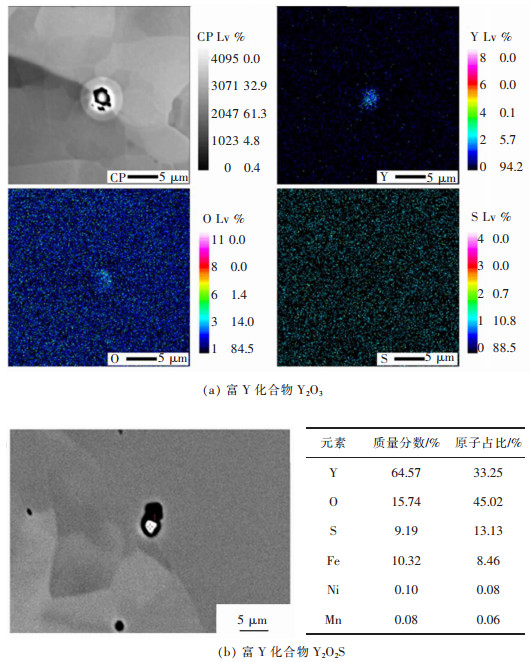
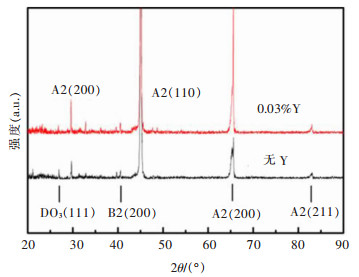
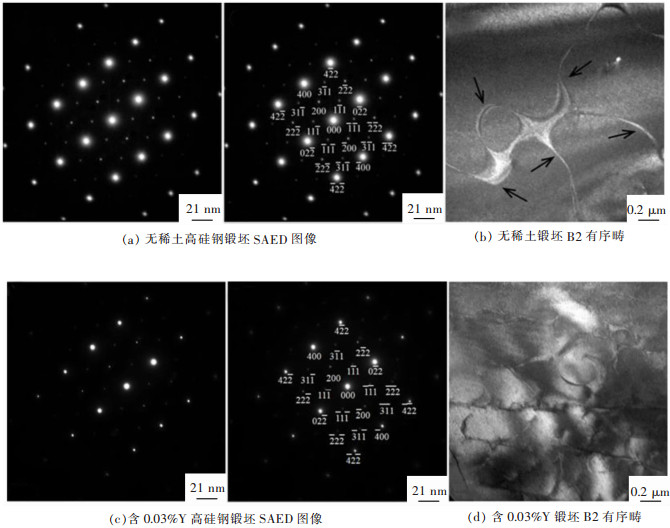
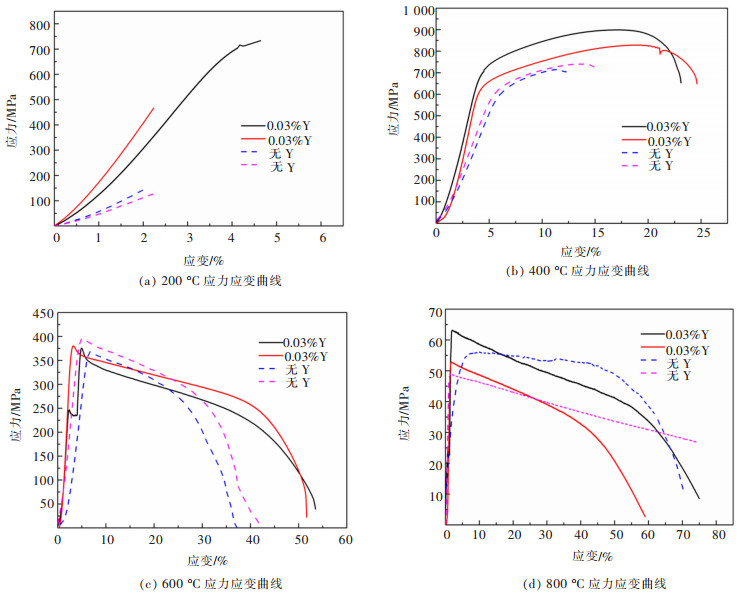
摘要: 6.5%Si高硅钢具有高磁导率、低铁损、几乎为零的磁致伸缩等优良软磁性能, 具有广阔的应用前景。然而, 室温硬脆的B2和DO3有序结构出现阻碍了其生产和应用。文章研究了在200~800 ℃下, 稀土Y对6.5%Si高硅钢拉伸性能的影响, 利用EBSD、EPMA、XRD和TEM等技术研究了稀土Y对6.5%Si高硅钢显微组织及有序结构的影响。结果表明, 细小的Y2O3和Y2O2S等稀土化合物能够作为有效形核剂细化组织; 稀土Y的加入不仅细化了有序畴, 而且降低了有序相含量、有序度和硬度; 200~600 ℃含0.03%Y高硅钢的断裂延伸率高于无稀土高硅钢, 200~400 ℃含0.03%Y高硅钢的抗拉强度高于无稀土高硅钢, 在6.5%Si高硅钢添加稀土Y造成晶粒细化和有序度降低是其韧塑性提高的重要原因。Abstract: Fe-6.5wt%Si alloy shows potential for a broad application with excellent soft magnetic properties including high permeability, low iron loss, and near-zero magnetostriction. However, brittle-ordered B2 and DO3 structures hinders its mass production and applications. In this work, the effect of Y element on the tensile properties of Fe-6.5wt%Si alloy at 200~800 ℃ was investigated. A detailed study of the Y element in the microstructure and ordered structure of Fe-6.5wt%Si was carried out by EBSD, EPMA, XRD and TEM. Results showed that Y2O3 and Y2O2S precipitates as the efficient nucleation agents refined the microstructures. Adding Y not only refined the ordered domains, but also reduced the ordered phase contents, ordered degrees, and hardness. The fracture elongation of high silicon steel with 0.03% Y at 200~600 ℃ was higher than that of high silicon steel without Y. The tensile strength of high silicon steel containing 0.03% Y was higher than that of high silicon steel without Y at 200~400 ℃. The grain refinement and ordered degree reduction caused by the addition of rare earth Y in Fe-6.5wt%Si alloy were the important reasons for its improvement in ductility.
-
Keywords:
- Fe-6.5wt%Si alloy /
- rare earth /
- ordered degree /
- tensile property
-
CuCr合金具有较高的强度、硬度、导热导电能力和耐腐蚀性能,因此在电极材料、接触线、集成电路引线框架、连铸机结晶器内衬等方面有着广泛应用.其中,Cr含量10 %~50 %的CuCr合金具有优良的耐电压能力、抗熔焊性能和截流水平,是理想的中高压大功率真空开关触头材料,在国内外获得了广泛的应用[1-3]. Cu和Cr室温下的固溶度很小,合金显微组织为细小的富铬相晶粒弥散分布于富铜相基体中,较小的富Cr相晶粒有利于提升合金的耐电压和截流性能.目前工业上应用较为广泛的生产方法是以纯铜粉和纯铬粉为原料通过烧结或者熔渗制备CuCr合金,但富铬相尺寸受到原料铬粉粒度限制.真空自耗法可以获得Cr颗粒细小且分布均匀的合金,但生产流程长,设备复杂,成本高.由于Cu和Cr的熔点与密度均有较大差别,且CuCr合金是一种难混溶合金,二元系中存在两液相共存区,铸造过程中Cr粒子析出后会在重力作用下迅速上浮,导致两相分离,因此常规熔铸工艺制备CuCr合金较为困难,目前基本只用于CuCr25合金的生产.
自蔓延冶金法具有流程短,设备投资小,生产成本低等特点.张廷安等[4-6]提出以氧化物为原料,利用自蔓延冶金法制备CuCr合金,该方法包括3个关键步骤,首先通过铝热反应获得高温熔体,然后将熔体转移到坩埚中进行熔渣精炼,最后对其进行快速冷却以获得合金铸锭.利用该工艺已经成功制备出Cr含量15 %~40 %的CuCr合金,其富铬相尺寸在20 μm左右,接近真空自耗法产品.在铝热还原过程中将有大量的Al2O3产生,Al2O3将和造渣剂、未反应的氧化物(主要是Cr2O3)一起形成熔渣,渣中Al2O3含量较高,凝固温度高、流动性较差.增加造渣剂添加量可以改善熔渣的性能,但同时会降低体系的反应温度,反而对渣金分离过程带来不利影响.由于该工艺中铝热反应产生的熔渣直接用作第2步中的精炼渣,成分受到一定的限制,故需要综合考虑两方面的影响,选取适当的成分,使冶炼渣具有适当的性能且不会过多地降低反应温度.
Cr2O3相对不易还原,通常会有少量进入冶炼渣,同时,Al2O3和Cr2O3均有较高的熔点,在铝热反应获得的高温熔体转移到坩埚内时,会有部分Al2O3和Cr2O3颗粒夹杂在金属熔体内,在熔渣精炼过程中上浮并被熔渣吸收,改变熔渣的成分,对熔渣性能有不利影响[7, 8].而熔渣熔点、黏度、密度和表面张力等热物理性能的变化,会影响到渣金分离过程和熔渣精炼效果[9-12].因此,文中考察了Al2O3和Cr2O3对铜铬合金冶炼渣性能的影响,为CuCr合金自蔓延冶金法制备提供了理论依据.
1 实验方法与步骤
1.1 实验原料
实验中所使用的熔渣均以分析纯化学试剂配制,为脱除试剂中的水分和挥发性杂质,所有试剂均在马弗炉中进行高温焙烧预处理,其中CaF2经500 ℃、6 h处理,其它试剂经1 000 ℃、4 h处理,处理后的试剂装瓶放入干燥器内储存.根据前期研究成果,选择w(CaF2)= 20 %、w(CaO): w(Al2O3)=1:2的熔渣(CAF5)为基础渣系,分别向其中添加质量分数为2.5 %~10 %的Al2O3和Cr2O3,以考察Al2O3和Cr2O3对冶炼渣性能的影响,渣组成配比如表 1所列.实验前,按照预定成分称取试剂,然后在混料罐中混合均匀.
表 1 渣系组成配比/(质量分数,%)Table 1. Composition of slags /(massfraction, %)
1.2 实验方法
采用RTW-10型熔体物性测定仪(如图 1所示)进行熔渣性能测试实验,熔渣的黏度使用内旋转柱体法测定,密度采用阿基米德法测定,表面张力采用拉筒法测定,凝固温度利用Seetharaman的方法[13-15]由熔渣的黏度温度曲线来估算.实验中所使用的坩埚为石墨质,探头均为钼质.取140 g混合好的渣料放入尺寸为ø40 mm×70 mm的高纯石墨坩埚中在二硅化钼电炉中随炉升温至1 650 ℃,保温30 min以保证熔渣熔化充分且均匀混合,用直径为8 mm的铁棒蘸出多余的熔渣,将熔池深度调整为40 mm,测量熔渣的密度、表面张力和黏度,随后以-3 ℃/min的速度降温并测定熔渣的黏度-温度曲线并计算其凝固温度.
2 结果与讨论
2.1 Al2O3和Cr2O3对冶炼渣黏度的影响
向冶炼渣中添加不同含量的Al2O3和Cr2O3时熔渣的黏度-温度曲线如图 2所示.由图 2中可以看出,由于氧化铝含量较高,自蔓延冶炼渣呈现出典型的“短渣”特性,所有渣系的黏度-温度曲线上均有明显的拐点,在拐点温度以上时黏度随温度的下降缓慢增加,而温度下降到拐点温度之后黏度开始急剧上升,并在很小的温度范围内完全凝固,这是铝酸盐熔渣的典型特征.在熔融状态下的冶炼渣黏度较小,流动性很好,所有渣系在1 650 ℃时的黏度均小于0.15 Pa·s;随着Al2O3和Cr2O3添加量的增加,渣系黏度和拐点温度均上升.
图 3显示了1 650 ℃时渣系的黏度随Al2O3和Cr2O3添加量的变化,由图 3中可以看出,随着Al2O3和Cr2O3含量的增加,熔渣的黏度逐渐增大,但与Al2O3相比,Cr2O3对熔体黏度有更大的影响.根据目前对熔融氧化铝和铝酸盐熔体结构的研究结果[16-19],Al2O3在铝酸盐熔体中与硅酸盐熔体中的SiO2类似,通过Al-O-Al键聚合成网状结构,随碱性氧化物的增加,Al的配位数降低,复杂熔体结构解聚.因此,Al2O3含量的增加会提高熔体的聚合程度,从而导致熔融炉渣黏度的增大.作为过渡金属氧化物,Cr2O3在铝酸盐熔渣中可以起到与Al2O3类似的作用,提高渣系的聚合程度,而且Cr3+的离子半径(0.061 5 nm)比Al3+的离子半径(0.053 5 nm)更大,会对熔体中离子的自由移动造成较大的阻碍,从而提高渣系的黏度;同时,过多的Cr2O3还可能导致渣中高熔点铬酸盐相的生成,进一步提高熔体的黏度[20-22].
熔渣黏度会影响金属液滴在熔渣中的沉降速度,从而影响渣金分离效果,同时还会影响夹杂物在熔渣中的溶解速度. Al2O3和Cr2O3添加量的提高均会增加熔渣的黏度,对冶炼渣的性能有不利影响;由于Cr2O3对熔体黏度的影响更大,故控制渣中Cr2O3的含量更低,有利于提高冶炼渣的渣金分离性能和夹杂物溶解性能.
2.2 Al2O3和Cr2O3对冶炼渣凝固温度的影响
由熔渣的黏度-温度曲线计算其黏度活化能,再由黏度活化能对温度的二阶偏导数来估算冶炼渣的凝固温度,结果如图 4所示.由图 4中可以看出,随着Al2O3和Cr2O3含量的增加,熔渣的凝固温度均逐渐升高,且与Cr2O3相比,Al2O3对熔体凝固温度的影响更加剧烈.由于Al2O3和Cr2O3的熔点均较高,分别为2 054 ℃和2 435 ℃,它们的含量提高会降低熔体中造渣剂CaO和CaF2的含量,从而引起冶炼渣凝固温度的提高,给冶炼渣的性能带来不利影响.
2.3 Al2O3和Cr2O3对冶炼渣密度的影响
1 650 ℃时冶炼渣密度的测量结果如图 5所示,由图 5中可以看出,成分对冶炼渣密度影响不大,在2.654~2.698 g/m3之间,Al2O3含量的提高会使熔体的密度上升,Cr2O3含量的提高则会使熔体的密度先升高后降低,在w(Cr2O3)=5 %时有极大值. Al2O3和Cr2O3的密度分别为3.97 g/cm3和5.21 g/cm3,属于密度较大的组分,因而它们含量的升高会使得熔渣密度增大.由于Cr2O3是两性氧化物,可以进入熔融炉渣网状结构,提高熔体的聚合程度;而Cr3+的离子半径较大,在进入网状结构后可能会导致结构单元体积变大,这可能是Cr2O3含量的进一步提高导致熔渣密度降低的原因.金属熔体和熔融炉渣间的密度差影响着金属熔滴在熔渣中的沉降速度,因此渣中Al2O3和Cr2O3含量的增加会提高熔渣的密度从而降低冶炼渣的渣金分离效果,但CuCr合金的密度较大,约为8 g/cm3,而成分变化引起的冶炼渣的密度变化值仅有0.044 g/cm3,因此熔渣密度变化对渣金密度差影响很小,对冶炼渣性能的影响不大.
2.4 Al2O3和Cr2O3对冶炼渣表面张力的影响
1 650 ℃时冶炼渣表面张力的测量结果如图 6所示,由图 6中可以看出,熔渣的表面张力在347~419 mN/m之间,Al2O3和Cr2O3含量的增加均会提高冶炼渣的表面张力,在含量不超过5 %时,Cr2O3的影响更加显著,含量在5 %以上时两者的影响相差不大. Al2O3和Cr2O3含量的提高一方面会提高渣系的聚合程度,使渣中复合阴离子增多,另一方面减少了渣中较强的表面活性物质F-离子的含量,从而引起熔渣的表面张力上升.冶炼渣表面张力越大,金渣黏附功越小,熔渣不容易被金属熔体卷入,有利于提高合金的洁净度.因此,Al2O3和Cr2O3含量的提高有助于改善冶炼渣的表面张力.
3 结论
1)自蔓延冶炼渣黏度随温度升高而下降,且属于典型的短渣,渣系流动性较好,1 650 ℃时黏度小于0.15 Pa·s;随Al2O3和Cr2O3含量提高,熔渣的黏度上升,Cr2O3含量对冶炼渣黏度的影响更大;凝固温度随Al2O3和Cr2O3含量提高而上升,Al2O3含量对冶炼渣凝固温度的影响更大;密度随Al2O3含量提高而上升,随Cr2O3含量提高而先上升后下降,在5 %时最大;表面张力随Al2O3和Cr2O3含量提高而上升,Cr2O3含量对表面张力的影响更大.
2)提高熔体中的Al2O3和Cr2O3含量可以改善自蔓延冶炼渣的表面张力,但对黏度和凝固温度有不利影响,前期研究中发现,熔渣的黏度和凝固温度对合金质量影响更大,因此Al2O3和Cr2O3含量对熔渣总体性能有不利影响.
-
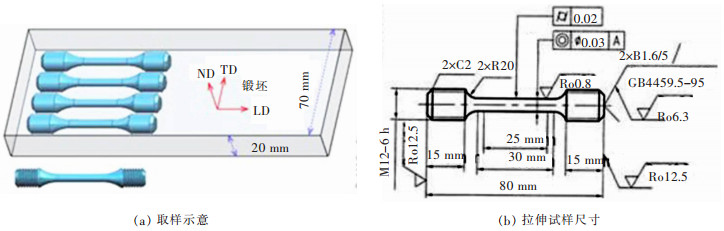
表 1 2种6.5 %Si高硅钢锻坯的化学成分
Table 1 Chemical composition content of two kinds of 6.5% Si high silicon steel forged slabs

-
[1] LIANG Y F, WANG S, QI J K, et al. Microstructure and properties of cost-effective Fe-6.5 wt% Si ribbons fabricated by melt-spinning[J]. Scripta Materialia, 2019, 163(2019): 107-110. http://www.sciencedirect.com/science/article/pii/S1359646219300028
[2] OUYANG G Y, CHEN X, LIANG Y F, et al. Review of Fe-6.5?%Si high silicon steel—A promising soft magnetic material for sub-kHz application[J]. Journal of Magnetism and Magnetic Materials, 2019, 481(2019): 234-250. http://www.sciencedirect.com/science/article/pii/S0304885318331330
[3] 杨经富, 张迎晖, 秦镜, 等. 成品厚度对高牌号无取向电工钢组织、织构和磁性能的影响[J]. 有色金属科学与工程, 2020, 11(3): 73-79. http://ysjskx.paperopen.com/oa/DArticle.aspx?type=view&id=202003010 [4] SHIN J S, BAE J S, KIM H J, et al. Ordering-disordering phenomena and micro-hardness characteristics of B2 phase in Fe-(5-6.5%) Si alloys[J]. Materials Science and Engineering: A, 2005, 407: 282-290. doi: 10.1016/j.msea.2005.07.012
[5] PENG M H, ZHONG Y B, ZHENG T X, et al. 6.5 wt% Si high silicon steel sheets prepared by composite electrodeposition in magnetic field[J]. Journal of Materials Science & Technology, 2018, 34(12): 2492-2497. http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFD&filename=CLKJ201812035
[6] HE X D, LI X, SUN Y. Microstructure and magnetic properties of high silicon electrical steel produced by electron beam physical vapor deposition[J]. Journal of Magnetism and Magnetic Materials, 2008, 320(3/4): 217-221.
[7] TIAN G K, BI X F. Fabrication and magnetic properties of Fe-6.5% Si alloys by magnetron sputtering method[J]. Journal of Alloys and Compounds, 2010, 502(1): 1-4. doi: 10.1016/j.jallcom.2010.02.175
[8] YU H, BI X F. Magnetic and mechanical properties of the gradient FeSi alloys fabricated by magnetron sputtering[J]. Journal of Alloys and Compounds, 2015, 634: 83-86. doi: 10.1016/j.jallcom.2015.01.156
[9] WANG X L, LI H Z, LIU Z Y, et al. Effect of cooling rate on bending behavior of 6.5. % Si electrical steel thin sheets fabricated by strip casting and rolling[J]. Materials Characterization, 2016, 111: 67-74. doi: 10.1016/j.matchar.2015.11.008
[10] LIANG Y F, YE F, LIN J P, et al. Effect of heat treatment on mechanical properties of heavily cold-rolled Fe-6.5% Si alloy sheet[J]. Science China Technological Sciences, 2010, 53(4): 1008-1011. doi: 10.1007/s11431-010-0125-1
[11] ZHANG Z H, WANG W P, FU H D, et al. Effect of quench cooling rate on residual stress, microstructure and mechanical property of an Fe-6.5Si alloy[J]. Materials Science and Engineering: A, 2011, 530: 519-524. doi: 10.1016/j.msea.2011.10.013
[12] MO Y K, ZHANNG Z H, FU H D, et al. Effects of deformation temperature on the microstructure, ordering and mechanical properties of Fe-6.5% Si alloy with columnar grains[J]. Materials Science and Engineering: A, 2014, 594: 111-117. doi: 10.1016/j.msea.2013.11.024
[13] XIE J X, FU H D, ZHANG Z H, et al. Deformation twinning feature and its effects on significant enhancement of tensile ductility in columnar-grained Fe-6.5. %Si alloy at intermediate temperatures[J]. Intermetallics, 2012, 23: 20-26. doi: 10.1016/j.intermet.2011.12.011
[14] FU H D, ZHANG Z H, PAN H J, et al. Warm/cold rolling processes for producing Fe-6.5% Si electrical steel with columnar grains[J]. International Journal of Minerals, Metallurgy and Materials, 2013, 20(6): 535-540. doi: 10.1007/s12613-013-0762-z
[15] WANG S, JIANG Y M, LIANG Y F, et al. Magnetic properties and core loss behavior of Fe-6.5. %Si ribbons prepared by melt spinning[J]. Advances in Materials Science and Engineering, 2015, 2015: 1-6. http://www.researchgate.net/publication/276322823_Magnetic_Properties_and_Core_Loss_Behavior_of_Fe-65wtSi_Ribbons_Prepared_by_Melt_Spinning
[16] OUYANG G Y, JENSEN B, TANG W, et al. Effect of wheel speed on magnetic and mechanical properties of melt spun Fe-6.5% Si high silicon steel[J]. AIP Advances, 2018, 056111: 1-6. http://adsabs.harvard.edu/abs/2018AIPA....8e6111O
[17] MAO W M, YANG P. Influence of structure transition on plastic behaviors of iron based ordered alloys[J]. Science China Technological Sciences, 2012, 55(10): 2920-2925. doi: 10.1007/s11431-012-4947-x
[18] KIM K N, PAN L M, LIN J P. The effect of boron content on the processing for Fe-6.5% Si electrical steel sheets[J]. Journal of Magnetism and Magnetic Materials, 2004, 277(3)?: 331-336.
[19] CHEN W S, LIU J, ChENG Z Y, et al. Effect of chromium on microstructure, ordered phase and magnetic properties of Fe-6.5% Si alloy[J]. Materials Today: Proceedings, 2015, 2: 314-318. doi: 10.1016/j.matpr.2015.05.044
[20] YANG K, LIANG Y F, YE F, et al. Texture evolution of Nb microalloyed Fe14Si2 high silicon steel during warm rolling[J]. Acta Metallurgica Sinica, 2013, 49(11): 1411-1415. doi: 10.3724/SP.J.1037.2013.00492
[21] SCHULTE M, STEENTJES S, LEUNING N, et al. Effect of manganese in high silicon alloyed non-oriented electrical steel sheets[J]. Journal of Magnetism and Magnetic Materials, 2019, 477: 372-381. doi: 10.1016/j.jmmm.2018.07.025
[22] KIM K N, PAN L M, LIN J P, et al. The effect of boron content on the processing for Fe-6.5wt% Si electrical steel sheets[J]. Journal of Magnetism & Magnetic Materials, 2004, 277(3): 331-336. http://www.sciencedirect.com/science/article/pii/S0304885303009284
[23] PAN F, ZHANG J, CHEN H, et al. Effects of rare earth metals on steel microstructures[J]. Materials, 2016, 9(6): 417. doi: 10.3390/ma9060417
[24] LI H Z, LIU H T, WANG X L, et al. Effect of cerium on the as-cast microstructure and tensile ductility of the twin-roll casting Fe-6.5 % Si alloy[J]. Materials Letters, 2016, 165: 5-8. doi: 10.1016/j.matlet.2015.11.100
[25] YU X, ZHANG Z H, XIE J X. Effects of rare earth elements doping on ordered structures and ductility improvement of Fe-6.5 % Si alloy[J]. Materials Letters, 2016, 184: 294-297. doi: 10.1016/j.matlet.2016.08.074
[26] YU X, ZHANG Z H, XIE J X. Microstructure, Ordered structure and warm tensile ductility of Fe-6.5% Si alloy with various ce Content[J]. Acta Metallurgica Sinica, 2017, 53(8): 927-936. http://en.cnki.com.cn/Article_en/CJFDTotal-JSXB201708004.htm
[27] GAO X, REN H, LI C, WANG H, et al. First-principles calculations of rare earth (Y, La and Ce) diffusivities in bcc Fe[J]. Journal of Alloys and Compounds, 2016, 663: 316-320. doi: 10.1016/j.jallcom.2015.12.129
[28] GAO X, REN H, WANG H, et al. Activity coefficient and solubility of yttrium in Fe-Y dilute solid solution[J]. Journal of Rare Earths, 2016, 34(11): 1168-1172. doi: 10.1016/S1002-0721(16)60149-7
[29] 杨全海, 杨吉春, 丁海峰, 等. 稀土管线钢中夹杂物热力学分析及实验研究[J]. 稀土, 2018, 39: 96-101. https://www.cnki.com.cn/Article/CJFDTOTAL-XTZZ201802013.htm [30] MATSUMURA S, TANAKA Y, KOGA Y. Concurrent ordering and phase separation in the vicinity of the metastable critical point of order-disorder transition in Fe-Si alloys[J]. Materials Science and Engineering: A, 2001, 312(1/2): 284-292. http://www.sciencedirect.com/science/article/pii/S0921509300018748
[31] JUNG H, KIM S B, KIM J B, et al. Effects of anti-phase boundary on the iron loss of grain oriented silicon steel[J]. ISIJ International, 2011, 51(6): 987-990. doi: 10.2355/isijinternational.51.987
[32] QIN J, YUE Y, ZHANG Y H, et al. Comparison between strong η-fiber-oriented high-silicon steel and grain-oriented high-silicon steel on magnetic properties[J]. Journal of Magnetism and Magnetic Materials, 2017, 439: 38-43. doi: 10.1016/j.jmmm.2017.05.006
[33] YU H Y, MING K S, WU H C, et al. Ordering suppression and excellent ductility in soft-magnetic Fe-6.5% Si sheet by Hf addition[J]. Journal of Alloys and Compounds, 2018, 766: 186-193. doi: 10.1016/j.jallcom.2018.06.343
[34] LI H Z, LIU Z Y. Tensile properties of strip casting 6.5% Si steel at elevated temperatures[J]. Materials Science and Engineering: A, 2015, 639: 412-416. doi: 10.1016/j.msea.2015.05.057
-
期刊类型引用(9)
1. 钱康乐,彭海益,何代华,姚晓刚,林慧兴. h-BN对超低介电损耗COC/h-BN复合材料导热性能、介电性能的影响. 有色金属材料与工程. 2025(01): 67-74 .  百度学术
百度学术
2. 夏春勇,杨长龙,多俊龙,豆志河,张廷安,韩金儒,安旺. 中高压开关用Cu基触头材料研究进展. 特种铸造及有色合金. 2024(05): 598-605 .  百度学术
百度学术
3. 朱炳耀,贾小波. 超声振动原位Al_2O_(3(p))/7075汽车零件合金组织与耐腐蚀性能研究. 有色金属科学与工程. 2023(04): 511-517 .  本站查看
本站查看
4. 孙澄川,卢静,解路,吴应东,陈东,但幸东. 冷喷涂制备铜基合金涂层研究进展. 材料保护. 2022(07): 165-176 .  百度学术
百度学术
5. 陈婷,汤惠东,邵川,张筱君,江伟辉. 非水解溶胶-凝胶法制备Cr掺杂α-Al_2O_3红色色料. 稀有金属. 2021(08): 989-997 .  百度学术
百度学术
6. 邹宗轩,刘政军,韩旭. W对Fe-Cr-C-W-B系堆焊合金组织和性能的影响. 焊接学报. 2021(07): 91-96+104 .  百度学术
百度学术
7. 梁炳联. 关于自蔓延冶金法制备粉体及合金的研究进展. 冶金与材料. 2020(01): 29+31 .  百度学术
百度学术
8. 祝家祺,谭谆礼,张敏,王军祥,白秉哲,翁宇庆. 钒和铬对贝氏体车轮钢回火组织与性能的影响. 稀有金属. 2020(09): 957-966 .  百度学术
百度学术
9. 李宁. 基于冶金企业的地下金属管线方位测量控制方法研究. 世界有色金属. 2019(15): 179+181 .  百度学术
百度学术
其他类型引用(0)



 下载:
下载: