Effect of brine-ammonia reverse precipitation on crystal growth of magnesium hydroxide
-
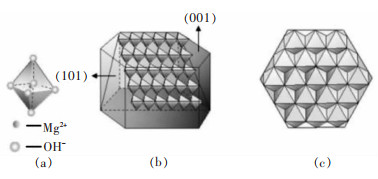
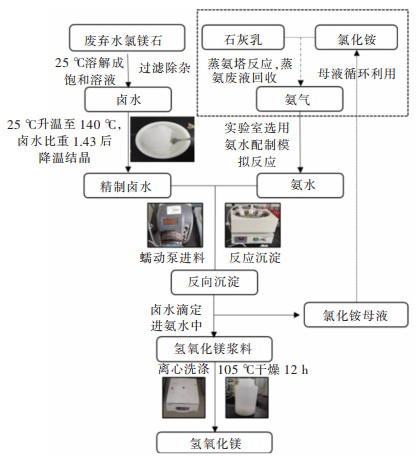
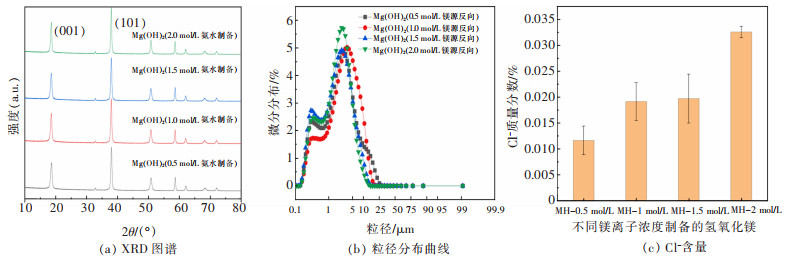
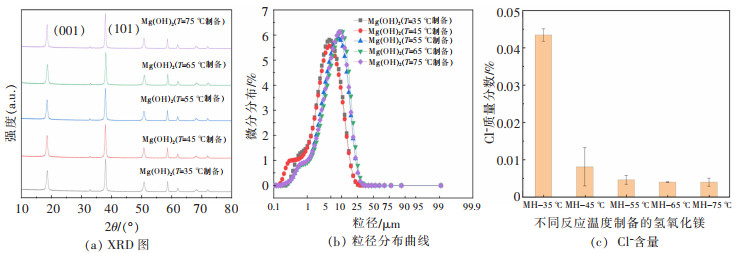
摘要: 以盐湖卤水和氨水为原料,采用反向沉淀法合成六角片状氢氧化镁。研究了Mg2+离子浓度、反应温度、进料速度、陈化时间等反应条件对氢氧化镁结晶度、粒径和氯根含量的影响,综合反映氢氧化镁晶面生长情况。通过SEM、XRD、激光粒度分析仪进行了表征分析。结果表明,Mg2+离子浓度和反应温度的提高,有利于氢氧化镁(001)晶面的生长。随着Mg2+离子浓度的增大、陈化时间的延长,氢氧化镁中的氯根含量增大。氢氧化镁的粒径受反应条件及晶面生长、极性影响较大。通过盐湖卤水-氨法反向沉淀能够制备(001)晶面尺寸为13.1~19.4nm、粒径D50为1.64~9.32μm,Cl-含量<0.1%的六角片状氢氧化镁。可望为调控盐湖水氯镁石制备氢氧化镁晶面生长提供科学依据,并为利用盐湖资源制备高纯、高附加值的氢氧化镁功能材料提供借鉴。Abstract: Reverse precipitation method was applied to synthesize hexagonal flaky magnesium hydroxide by using brine of salt lake and ammonia as raw materials. The effects of magnesium ion concentration, reaction temperature, feed rate and aging time on the crystallinity, particle size and chloride content of magnesium hydroxide were studied, and the crystal plane growth of magnesium hydroxide was comprehensively reflected. Herein, characterization analysis of the samples was carried out by SEM, XRD and laser particle size analyzer. The results showed that, with the increase of Mg2+ ion concentration and reaction temperature, it was favorable for the crystal plane growth of magnesium hydroxide(001). The increase of Mg2+ ion concentration and the prolongation of aging time made the chloride content of magnesium hydroxide increase. Its particle size was greatly affected by reaction conditions, crystal plane growth and polarity. Hexagonal flaky magnesium hydroxide with (001) crystal face size of 13.1-19.4 nm, particle size D50 of 1.64-9.32 μm, and chloride content < 0.1% could be prepared by brine-ammonia reverse precipitation. These results could not only provide a scientific basis for regulating the growth of magnesium hydroxide crystal planes prepared from bischofite in salt lakes, but also offer a useful reference for using salt lake resources to prepare high-purity, high-value-added functional materials of magnesium hydroxide.
-
0 引言
我国拥有极其丰富的铝矿资源,铝合金型材已被各领域广泛应用.铝合金型材生产过程中最主导技术以及关键的核心环节是挤压成型[1],型材质量的好坏决定于模具设计结构与挤压工艺参数,如何选择好的挤压工艺参数和延长挤压模具的寿命已成为各行业亟待解决的问题.型材挤压工艺参数设计是挤压设计中最为关键的要领之一,它主要包括挤压比、挤压速度、挤压温度等要素.挤压工艺参数设计合理与否直接关系到后续型材产品的表面质量以及挤压力大小,以往在对挤压工艺参数的选择上往往是通过反复试用以及经验获得,本文主要是通过对型材挤压过程进行仿真模拟,在试模挤压前利用仿真模拟技术对挤压加工进行模拟,通过改变挤压工艺参数的数值,计算获得实验现场无法获得的型材物理性能.
针对挤压过程的仿真模拟国内外做过类似的研究[2-5],本次研究就是以某6063空心铝型材挤压加工为研究为对象,以Altair Hyperxtrude软件为仿真模拟载体,基于Taguchi分析方法,获得挤压该型材时的最佳工艺参数配比,为企业生产提供参考,从而提高生产效率.
1 实验研究方法
1.1 Altair Hyperxtrude挤压仿真模拟软件的简介
铝型材挤压成型在铝型材加工领域是一个高压高温以及复杂的非线性、大变形的热-力耦合的塑性成型加工过程,Altair Hyperxtrude是目前全球唯一专业的铝型材挤压仿真模拟软件,同时可以对模具结构进行优化的软件,可以进行正向挤压和反向挤压分析,求解类型有瞬态和稳态2种,hyerxtrude采用了ALE算法,ALE算法是以非线性的纳维叶-斯托克斯方程作为控制方程,有连续性、动量守恒和能量守恒3个基本方程控制[6-9].
连续性方程:
$$ \frac{{\partial \rho }}{{\partial t}} + {c_i}\frac{{\partial \rho }}{{\partial {x_i}}} + \rho \frac{{\partial {v_i}}}{{\partial {x_i}}} = 0 $$ (1) 动量守恒方程:
$$ \rho \left( {\frac{{\partial {v_i}}}{{\partial t}} + {c_i}\frac{{\partial {v_i}}}{{\partial {x_i}}}} \right) = \frac{{\partial {\tau _{ji}}}}{{\partial {x_i}}} + {p_i} $$ (2) 能量守恒方程:
$$ \rho \left( {\frac{{\partial E}}{{\partial t}} + {c_i}\frac{{\partial E}}{{\partial {x_i}}}} \right) = \frac{{\partial {T_{ji}}{v_j}}}{{\partial {x_i}}} + {p_j}{v_j} + \frac{{\partial \left( {{K_{ij}}, {T_j}} \right)}}{{\partial {x_i}}} + {K_0} $$ (3) 式(1)~式(3)中:v为材料的位移;ρ为材料密度;τ为柯西应力;T为热力学温度;E为内能;Kij为热传导系数;K0为单位体积的热源;t为时间;pi为作用于物体上单位质量的体力;ci为物质点相对于网格点的运动速度,即为对流速度.
1.2 Taguchi试验研究方法简介
Taguchi试验设计主要的理论基础是概率论和数理统计,通过Taguchi设计理念可以减少试验次数,通过试验方案对比寻找最佳的工艺参数配比,从而降低成本、提高实际生产效率并获得表面质量和性能最佳的型材产品.Taguchi方法[10-12]利用正交表来选择试验条件和安排试验方案,它的最大优点就是利用最少的试验次数获得最佳的工艺参数,该设计方法是一种简单有效的方法,凭借其设计优点被广泛应用于工程、化工、加工等领域.如图 1所示描述了Taguchi试验设计的一般流程.
信噪比(S/N ratio)作为衡量质量特性的重要依据,用于系统和产品开发.本次研究采用信噪比(S/N ratio)来衡量5个挤压工艺参数对型材截面速度均方差、挤压力的影响规律以及对产品品质特性的影响规律,研究采用静态特性中的望小特性(smaller the better).为了更好地研究采用望小特性(smaller the better),对于望小质量特性服从Y~N(μ,σ2)分布,可将信噪比定义为S/N用η表示,即η=μ2/σ2,用信噪比来衡量产品特性的稳定性.为了使η更加接近于正态分布,使效应趋于线性可加性,将η值变成分贝(dB)值,即
$$ {\eta _{\rm{s}}} = 10{\rm{lg}}\eta $$ 望小特性希望Y越小越好,即可以认为μ2、σ2越小越好,所以可以将η的值变为
$$ \eta = \frac{1}{{{\mu ^2} + {\sigma ^2}}} $$ 即:$ S/N = {\eta _{\rm{s}}} = 10{\rm{lg}}\eta = 10{\rm{lg}}\left( {\frac{1}{{{\mu ^2} + {\sigma ^2}}}} \right) = {\rm{-}}10{\rm{lg}}\left( {{\mu ^2} + {\sigma ^2}} \right) $
另一种表述方式为:
$$ S/N = \eta = {\rm{ - }}10\lg \left( {\frac{{\rm{1}}}{N}\sum\limits_{i = {\rm{1}}}^N {X_{\rm{i}}^2} } \right) $$ (4) 式(4)中,Xi:表示第i次试验的试验值;i:表示试验的序号;N表示试验总次数.
2 挤压模型
本研究的对象是山东某厂生产的型号为Y8255铝合金型材,该型材截面较复杂,每处壁厚分布不均匀.由于型材截面复杂的分布,相应建立了挤压模具三维图,上模外径为510 mm,厚度为130 mm如图 2,在上模上设有分流孔、分流桥、模芯等,下模如图 3,设有模孔焊合深度为17 mm.
将建立好的上模和下模进行组装,首先将组装好的三维模型图导入Hyperxtrude进行数值模拟,图 4为数值模拟分析模型,然后进行前处理,包括几何处理、网格划分等,在对网格划分时应注意以下3点:①型材最薄截面处至少划分6层单元网格;②对工作带区域划分时,沿挤压正方向至少划分9层单元网格;③自由面和工作带上以三菱柱单元网格形式划分,网格划分的顺序按工作带-焊合室-坯料,其他部分网格以离工作带越远网格单元尺寸越大的规则划分.
3 Altair Hyperxtrude挤压模拟结果分析
上述为初始方案(棒料直径210 mm,棒料、模具、料筒预热温度分布为480 ℃、480 ℃、450 ℃,挤压速度为2 mm/s)挤压仿真结果,图 5~图 8分别示出了挤压型材各部分的流速以及流经各处的速度.理论上[13-15],坯料在工作带上的流速越均匀挤压制品端面越平齐,从而型材质量更好,从图 5可以看到在平模部分明显快于分流部分,并在壁厚小的地方流速更快,这是因为在平模处孔大供料快,而在分流部分相对供料较慢,由图 6可以明显反映供料速度,图 7是坯料通过分流孔的速度,也就是分流孔的供料速度,从图 7中可以看出在坯料流经各分流孔时的相对速度,通过合理对比,在分流孔分配上起到主导作用,在流速快的地方可以适当减少分流孔面积;其中图 8反映的是坯料流经工作带时的流速,可以看出在平模处的流速要稍高于分流部分的速度.
4 Taguchi试验方法分析
利用Hyperxtrude挤压专用模拟软件分别对试验直交表中32组不同的挤压工艺条件下的挤压过程进行仿真模拟,利用公式:$ VRD = \frac{{\sum\limits_{i = 1}^{\rm{n}} {\frac{{\left| {{v_i}{\rm{-}}\overline v } \right|}}{{\overline v }}} }}{n} $,(vi为型材截面上节点i处材料的流动速度;v所有考察节点的平均速度;n为考察节点的总数).求出每组中挤压型材截面的速度相对均差VRD,为了获得更接近实际型材截面的VRD,所选取型材截面的所有节点的流出速度.
通过模拟out文件获得挤压力大小,同时利用式(4)计算出VRD、挤压力的信噪比,计算结果如表 1.
表 1 因素水平表Table 1. Factors and levels of experiments挤压工艺参数 水平1 水平2 水平3 水平4 棒料直径A/mm 200 205 210 215 挤压速度B/mm.s-1 1.4 2.0 2.6 3.2 棒料预热温度C/℃ 460 470 480 490 模具预热温度D/℃) 450 460 470 480 料筒预热温度E/℃ 430 440 450 460 试验序号 VRD值 VRD的信噪比 挤压力/t 挤压力的信噪比 1 0.026 5 31.535 1 003.2 -60.028 2 0.020 7 33.681 1 057.0 -60.481 3 0.019 0 34.425 1 055.8 -60.471 4 0.020 9 33.597 1 039.0 -60.332 5 0.020 4 33.807 1 016.5 -60.142 6 0.017 3 35.239 1 113.4 -60.933 7 0.013 8 37.202 1 067.4 -60.567 8 0.013 5 37.393 1 097.5 -60.808 9 0.026 1 31.667 1 018.3 -60.158 10 0.029 9 30.487 1 160.0 -61.289 11 0.018 3 34.751 1 166.5 -61.338 12 0.018 4 34.704 1 232.3 -61.814 13 0.021 9 33.191 1 082.0 -60.685 14 0.020 6 33.723 1 215.2 -61.693 15 0.021 4 33.392 1 237.3 -61.850 16 0.013 6 37.329 1 294.1 -62.239 17 0.025 6 31.835 880.56 -58.895 18 0.020 4 33.807 1 016.3 -60.140 19 0.018 9 34.471 1 094.8 -60.787 20 0.024 9 32.076 1 149.8 -61.212 21 0.024 5 32.217 914.4 -59.223 22 0.016 3 35.756 1 043.2 -60.367 23 0.014 6 36.713 1 137.2 -61.117 24 0.013 9 37.140 1 179.7 -61.435 25 0.024 1 32.360 1 022.3 -60.192 26 0.020 3 33.850 1 086.7 -60.722 27 0.032 1 29.870 1 228.3 -61.786 28 0.026 3 31.601 1 204.3 -61.615 29 0.018 9 34.471 1 060.5 -60.510 30 0.020 7 33.681 1 169.0 -61.356 31 0.024 2 32.324 1 267.8 -62.061 32 0.015 8 36.027 1 300.1 -62.280 通过模拟每组工艺参数条件下的挤压过程,计算获得挤压型材截面速度均方差VRD和挤压力的信噪比,为了准确分析各工艺参数对型材的影响,利用直观分析原理算出不同水平条件下各组工艺参数的信噪比的平均值具体见表 2、表 3.
表 2 不同水平下VRD的平均信噪比Table 2. Average signal-to-noise-ratios of VRD under different levels水平 VRD的平均信噪比 A B C D E 1 33.178 32.635 32.620 34.043 33.713 2 35.683 33.778 34.074 33.192 34.938 3 32.411 34.144 35.007 34.358 33.427 4 34.267 34.983 33.840 33.948 33.463 表 3 不同水平下挤压力的平均信噪比Table 3. Average signal-to-noise-ratios of extrusion force under different levels水平 挤压力的平均信噪比 A B C D E 1 -60.293 -59.979 -61.206 -60.983 -61.063 2 -60.574 -60.873 -61.007 -60.916 -60.957 3 -61.114 -61.247 -60.788 -60.863 -60.835 4 -61.584 -61.467 -60.566 -60.804 -60.711 将表 2、表 3不同水平因素的VRD和挤压力信噪比采用直线图的形式表示出来,结果如图 9、图 10,从图 9、图 10中可以看出不同挤压工艺参数对型材截面速度和挤压力有不同的影响规律,根据经验及理论知识可知:型材流经模具出口处的速度越均匀则型材表面质量越好、型材表面缺陷越少;挤压型材所需挤压力越小,挤压所需能耗越小,挤压过程中模具磨损量也越小.因此本研究目标是减小VRD和降低挤压力,故采用信噪比中的望小特性来评价指标的优劣.原理为:信噪比越大,目标变化越小,结果越好.
5 结论
以Hyperxtrude软件为模拟载体,基于Taguchi试验分析方法对模拟结果进行计算分析,并由直线趋势图以及望小特性信噪比原理得出以下结论:
1)对于型材截面流动均匀程度指标参数,最佳的挤压工艺参数组合为A2B4C3D3E2,即挤压棒料外径为205 mm、挤压垫速度3.2 mm/s、棒料预先加热温度为480 ℃、挤压模具预先加热温度为470 ℃、挤压筒预先加热温度为440 ℃.
2)对于挤压力指标参数,最佳工艺参数组合为A1B1C4D4E4,即棒料外径为200 mm、挤压垫速度为1.4 mm/s、棒料预先加热温度为490 ℃,挤压模具预先加热温度为480 ℃、挤压筒预先加热温度为460 ℃.
3)在实际生产厂家推荐以及经验制定的挤压参数范围内,改变挤压工艺参数对型材截面速度和挤压力有很大影响,其中改变棒料直径、挤压速度和棒料预热温度对其影响最大,可以通过改变其参数来控制型材质量.
6 结论验证
以图 2、图 3模具构造原理加工出实际挤压模具,以上述获得的最佳方案A2B4C3D3E2(型材在模具出口处的流速越均匀型材发生扭拧、翘曲等缺陷就越小)设置挤压工艺参数,首先通过Hyperxtrude进行仿真模拟,获得型材理论形变.然后以上述参数进行实际挤压设计,由华南某厂协助完成试模,选择的挤压设备为1 800 t卧式挤压机如图 11,通过试模获得实际型材制品如图 12.通过对型材制品与有限元模拟结果进行分析,可以得出两者变形趋势较一致,最终获得的型材表面光滑平整,没有出现因过烧而引起的表面斑点;纵向也较平直,没有出现因流速不均匀而引起的弯曲、扭拧、翘曲等宏观缺陷.
-
表 1 不同镁离子浓度制备的氢氧化镁晶体特征参数(T=45 ℃,V=5 mL/min,t=0.5 h)
Table 1 The crystal characterization parameters of magnesium hydroxide prepared with different concentrations of magnesium ions (T=45 ℃, V =5 mL/min, t=0.5 h)

表 2 不同反应温度制备的氢氧化镁晶体特征参数(C(Mg2+)=1 mol/L、V=5 mL/min、t=0.5 h)
Table 2 The crystal characteristic parameters of magnesium hydroxide prepared at different reaction temperatures (C(Mg2+)=1 mol/L, V=5 mL/min, t=0.5 h)

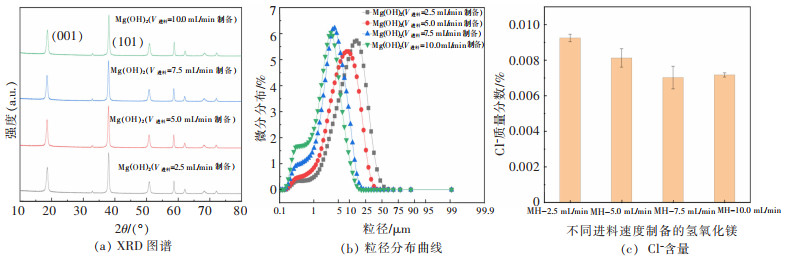
表 3 不同进料速度制备的氢氧化镁晶体特征参数(C(Mg2+)=1 mol/L,T=45 ℃,t=0.5 h)
Table 3 Crystal characteristic parameters of magnesium hydroxide crystal surface prepared at different feeding rates(C(Mg2+)=1 mol/L, T=45 ℃, t=0.5 h)

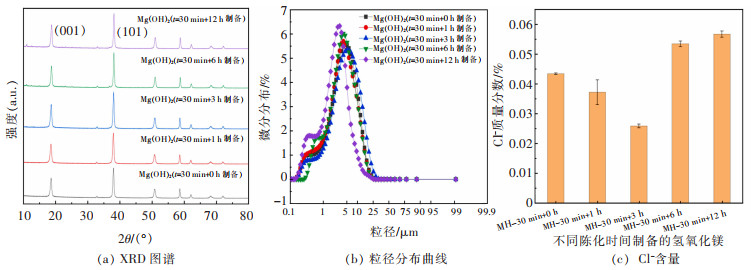
表 4 不同陈化时间制备的氢氧化镁晶体特征参数(C(mg2+)=1 mol/L,T=45 ℃,V=5 mL/min)
Table 4 Crystal characteristic parameters of magnesium hydroxide crystal surface prepared by different aging time(C(mg2+)=1 mol/L, T=45 ℃, V=5 mL/min)

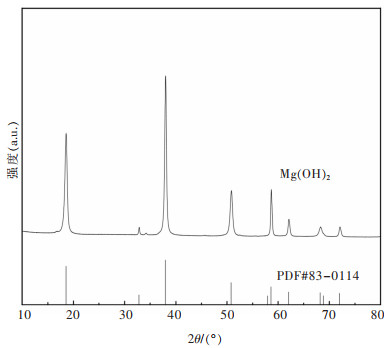
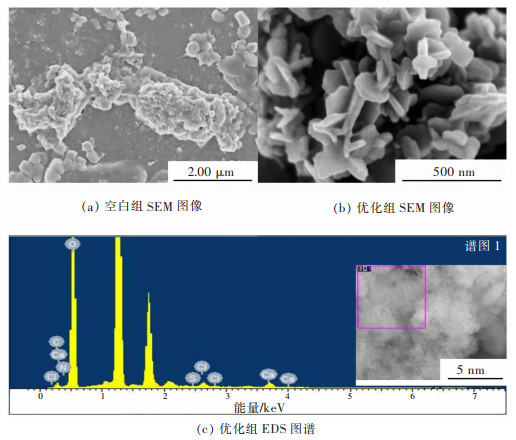
表 5 氢氧化镁晶体特征参数(C(Mg2+)=1.5 mol/L、T=65 ℃、V=5 mL/min、t=1 h)
Table 5 Crystal characteristic parameters of magnesium hydroxide crystal surface(C(Mg2+)=1.5 mol/L, T=65 ℃, V=5 mL/min, t=1 h)

-
[1] 李武, 董亚萍, 宋彭生, 等. 盐湖卤水资源开发利用[M]. 北京: 化学工业出版社, 2012. [2] 熊福军, 王肖虎, 张许, 等. 探索青海察尔汗盐湖老卤资源梯度开发[J]. 广东化工, 2022, 49(6): 158-161. https://www.cnki.com.cn/Article/CJFDTOTAL-GDHG202206071.htm [3] 吴礼定, 曾波. 钾肥副产镁资源制备氢氧化镁的生产技术[J]. 盐业与化工, 2012, 41(6): 26-30. https://www.cnki.com.cn/Article/CJFDTOTAL-HHYH201206010.htm [4] 王超超. 水氯镁石制备高纯氧化镁和碱式硫酸镁水泥研究[D]. 太原: 山西大学, 2020. [5] ABINAYA S, KAVITHA H P, PRAKASH M, et al. Green synthesis of magnesium oxide nanoparticles and its applications: a review[J]. Sustainable Chemistry and Pharmacy, 2021, 19: 100368. doi: 10.1016/j.scp.2020.100368
[6] 万兆源, 周桓. 水氯镁石制备金属镁的过程集成与能量分析[J]. 过程工程学报, 2020, 20(5): 609-618. https://www.cnki.com.cn/Article/CJFDTOTAL-HGYJ202005015.htm [7] 毕秋艳, 党力, 曹海莲, 等. 青海盐湖镁资源开发与利用研究进展[J]. 盐湖研究, 2022, 30(1): 101-109. https://www.cnki.com.cn/Article/CJFDTOTAL-YHYJ202201012.htm [8] 申红艳, 刘有智, 马鹏程, 等. 不同制备方法对纳米氢氧化镁性能的影响[J]. 化工进展, 2016, 35(4): 1149-1153. https://www.cnki.com.cn/Article/CJFDTOTAL-HGJZ201604030.htm [9] 朱华兵. 热解-水合制备氢氧化镁阻燃剂关键技术研究[D]. 天津: 天津科技大学, 2016. [10] 邓信忠. 水氯镁石电沉积制备氢氧化镁及其改性研究[D]. 沈阳: 东北大学, 2017. [11] 张梦婷, 白丽梅, 马玉新, 等. 超声辅助水化条件对氢氧化镁分散性的影响[J]. 非金属矿, 2020, 43(5): 5-7. https://www.cnki.com.cn/Article/CJFDTOTAL-FJSK202005002.htm [12] DONG C X, CAIRNEY J, SUN Q J, et al. Investigation of Mg(OH)2 nanoparticles as an antibacterial agent[J]. Journal of Nanoparticle Research, 2010, 12(6): 2101-2109. doi: 10.1007/s11051-009-9769-9
[13] LIU X M, LIAO C Z, LIN L, et al. Research progress in the environmental application of magnesium hydroxide nanomaterials[J]. Surfaces and Interfaces, 2020, 21: 100701. doi: 10.1016/j.surfin.2020.100701
[14] 范慧. 氢氧化镁的有机功能化以及在不同基体中的阻燃性能[D]. 兰州: 西北师范大学, 2020. [15] 赵卓雅, 李祥高, 王世荣, 等. 六角片状氢氧化镁(001)晶面优先生长条件的研究[J]. 人工晶体学报, 2014, 43(7): 1611-1619. https://www.cnki.com.cn/Article/CJFDTOTAL-RGJT201407006.htm [16] 徐嘉欣, 颜粉鸽, 黄建翠, 等. 高分散型六角片状氢氧化镁的制备与表征[J]. 盐科学与化工, 2018, 47(3): 14-21. https://www.cnki.com.cn/Article/CJFDTOTAL-HHYH201803004.htm [17] PIPEROPOULOS E, SCIONTI G, ATRIA M, et al. Flame-retardant performance evaluation of functional coatings filled with Mg(OH)2 and Al(OH)3[J]. Polymers, 2022, 14(3): 372.
[18] XU J S, YANG H Y, LUO Z B, et al. Synergistic effects of core@double-shell structured magnesium hydroxide microcapsules on flame retardancy and smoke suppression in flexible poly(vinyl chloride)[J]. RSC Advances, 2022, 12(5): 2914-2927.
[19] 黄建翠. 水菱镁矿的综合利用--纳米化镁、纳米氢氧化镁的制备及在重金属废水处理中的应用[D]. 上海: 华东师范大学, 2020. [20] 闫旭. 基于连续反应器的氢氧化镁制备工艺研究[D]. 沈阳: 沈阳化工大学, 2021. [21] 范天博, 姜宇, 刘露萍, 等. 一步水热法合成六方片状氢氧化镁及其生长习性分析的研究[J]. 人工晶体学报, 2017, 46(12): 2319-2325. https://www.cnki.com.cn/Article/CJFDTOTAL-RGJT201712003.htm [22] 张婧. 氨法合成Mg(OH)2的晶面选择性调控及对刚果红的吸附性能研究[D]. 北京: 北京化工大学, 2021. [23] 高雪焘. 六方片状氢氧化镁的制备、改性与阻燃应用研究[D]. 太原: 山西大学, 2021. [24] 吴易梅, 孙玉柱, 路贵民, 等. 结晶-水热法制备六角片状氢氧化镁[J]. 高校化学工程学报, 2019, 33(2): 425-434. https://www.cnki.com.cn/Article/CJFDTOTAL-GXHX201902022.htm [25] ZHU H, LI L, CHEN W D, et al. Controllable synthesis of coral-like hierarchical porous magnesium hydroxide with various surface area and pore volume for lead and cadmium ion adsorption[J]. Journal of Hazardous Materials, 2021, 416: 125922.
[26] 赵娜. 纳米氢氧化镁的卤水-碱法合成与分析及重质氧化镁的制备[D]. 上海: 华东师范大学, 2015. [27] 徐贵钰, 殷海青, 马明鹏. 盐湖卤水氨法制备氢氧化镁工艺探究[J]. 贵州师范大学学报(自然科学版), 2021, 39(3): 48-52. https://www.cnki.com.cn/Article/CJFDTOTAL-NATR202103008.htm [28] 郑水林, 王彩丽, 李春全. 粉体表面改性[M]. 4版. 北京: 中国建材工业出版社, 2019. [29] 易求实. 反向沉淀法制备纳米Mg(OH)2阻燃剂的研究[J]. 化学试剂, 2001(4): 197-199. https://www.cnki.com.cn/Article/CJFDTOTAL-HXSJ200104002.htm [30] 郑敏珠, 卢晗锋, 刘华彦, 等. 滴定过程控制Mg(OH)2晶体粒径和形貌[J]. 人工晶体学报, 2008(5): 1249-1254. https://www.cnki.com.cn/Article/CJFDTOTAL-RGJT200805045.htm [31] 谷静维, 张保林, 程亮, 等. 双滴加-反向沉淀法制备纳米氢氧化镁[J]. 化工矿物与加工, 2013, 42(9): 12-15. https://www.cnki.com.cn/Article/CJFDTOTAL-HGKJ201309004.htm [32] 宋磊, 曹雨微, 王景凤, 等. 盐湖卤水制备工业级氢氧化镁和氧化镁中氯的存在形式研究[J]. 盐湖研究, 2021, 29(3): 90-97. https://www.cnki.com.cn/Article/CJFDTOTAL-YHYJ202103011.htm [33] 杨喜云, 于培峰, 徐徽, 等. 盐湖卤水制备棒状氢氧化镁[J]. 中南大学学报(自然科学版), 2014, 45(1): 25-32. https://www.cnki.com.cn/Article/CJFDTOTAL-ZNGD201401004.htm [34] 周永红, 范天博, 刘露萍, 等. 六方片状氢氧化镁的合成及其第一性原理分析[J]. 化工学报, 2016, 67(9): 3843-3849. https://www.cnki.com.cn/Article/CJFDTOTAL-HGSZ201609040.htm [35] 张子兴. 盐湖卤水净化除杂工艺研究[D]. 太原: 山西大学, 2008. [36] 陈建铭, 张兆震, 宋云华. 水热反应条件对氢氧化镁晶体微观内应变的影响[J]. 人工晶体学报, 2011, 40(2): 396-404. https://www.cnki.com.cn/Article/CJFDTOTAL-RGJT201102024.htm [37] 杨晨. 多晶相水合碳酸镁结晶生长过程调控研究[D]. 上海: 华东理工大学, 2013. [38] 董碧岚. 水氯镁石纯碱反应结晶-煅烧制备高纯氧化镁过程研究[D]. 上海: 华东理工大学, 2013. [39] PYE C C. An ab initio investigation of lithium ion hydration. Ⅱ. Tetra-versus hexacoordination and halide complexes[J]. International Journal of Quantum Chemistry, 2000, 76(1): 62-76.
[40] KIPOUROS G J, SADOWAY D R. A thermochemical analysis of the production of anhydrous MgCl2[J]. Journal of Light Metals, 2001, 1(2): 111-117.
[41] DE WOLFF P M, WALTER-LEVY L. The crystal structure of Mg2(OH)3(Cl, Br(H2O)4[J]. Acta Crystallographica, 1953, 6(1): 40-44.
[42] 于培峰. 盐湖卤水制备棒状氢氧化镁的工艺及基础研究[D]. 长沙: 中南大学, 2013.




 下载:
下载: