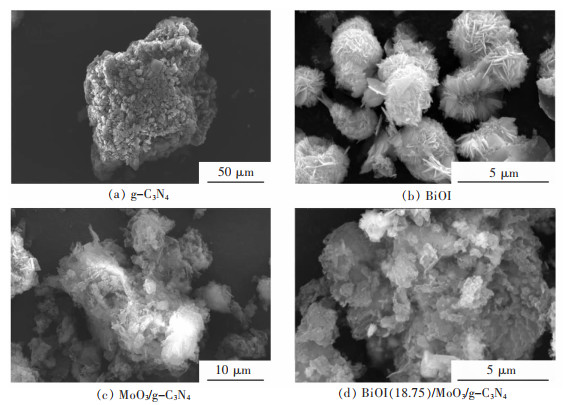
Construction of double Z-scheme heterojunction BiOI/MoO3/g-C3N4 and its photocatalytic performance
-
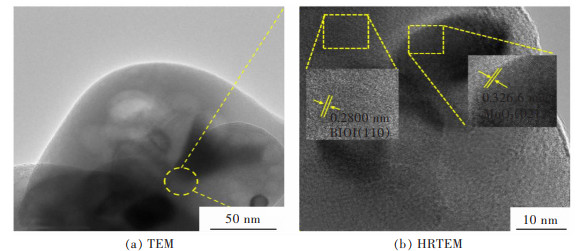
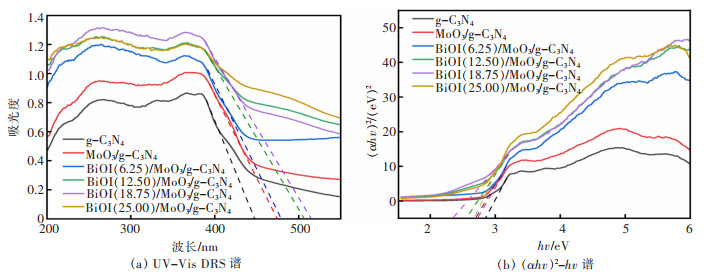
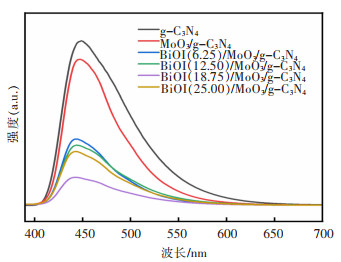
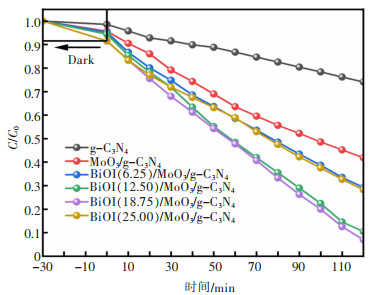
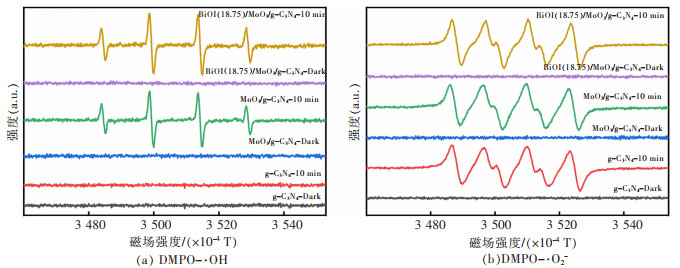
摘要: 通过简单的溶剂热法将半导体MoO3、BiOI与g-C3N4复合,构建双Z型异质结BiOI(x)/MoO3/g-C3N4(x=6.25%、12.50%、18.75%、25.00%,x为BiOI的质量分数)三元复合材料,从HRTEM结果可知样品出现了2种间距分别为0.28 nm和0.33 nm的晶格条纹,结合XRD表征结果可知分别属于BiOI(110)和MoO3(021)晶面,且g-C3N4是非晶态物质,由此表明BiOI/MoO3/g-C3N4复合材料成功复合。UV-Vis DRS分析表明复合样品的带隙变窄,光学响应范围增强,PL和光电化学测试表征说明异质结的存在有效延缓了电子和空穴的复合,在模拟太阳光条件下对染料甲基橙(MO)进行降解并研究其光催化活性,BiOI(18.75)/MoO3/g-C3N4具有较优异的光催化性能和光学稳定性,120 min对30 mg/L MO的降解率达94%,是纯g-C3N4的3.6倍。ESR表征说明BiOI/MoO3/g-C3N4光催化降解的主要活性物质组分为·OH和·O2-,并通过计算BiOI、MoO3和g-C3N4的价带和导带位置,表明3种物质是能带交错结构,推测出三元复合物形成双Z型异质结。BiOI/MoO3/g-C3N4可作为一种有效应用于有机染料污染物降解的可见光响应催化剂,具有应用前景。
-
关键词:
- MoO3 /
- BiOI /
- g-C3N4 /
- 光催化性能 /
- 双Z型异质结光催化剂
Abstract: Semiconductors MoO3, BiOI and g-C3N4 were compounded by a simple solvent thermal method, and finally, the ternary composite of double Z-scheme heterojunction BiOI(x)/MoO3/g-C3N4 (x=6.25%, 12.50%, 18.75%, 25.00%, x: mass percentage of BiOI) was constructed. From the HRTEM results, it can be seen that two types of lattice fringes with spacings of 0.28 nm and 0.33 nm appear in the sample. Combined with the XRD characterization results, it could be seen that they respectively belong to BiOI (110) and MoO3 (021) crystal planes, which thus indicats that the BiOI/MoO3/g-C3N4 composite was successfully compounded, since g-C3N4 is amorphous material. The results of UV-Vis DRS show the decreased band gap and greater optical response range of the composite samples. The PL and photoelectrochemical test characterizations illustrate that the presence of the heterojunction effectively delays the recombination of electrons and holes. The dye methyl orange (MO) was degraded under simulated sunlight conditions, and its photocatalytic activity was studied. BiOI(18.75)/MoO3/g-C3N4 had the best photocatalytic performance and optical stability, and the degradation rate of 30 mg/L MO was 94% for 120 min, which is 3.6 times that of pure g-C3N4. The ESR characterization indicates that the main active material components for the photocatalytic degradation of BiOI/MoO3/g-C3N4 were ·OH and ·O2-. By calculating the positions of the valence and conduction bands of BiOI, MoO3 and g-C3N4, it is shown that the three substances are band staggered structures, suggesting that the ternary complex forms a double Z-scheme heterojunction. BiOI/MoO3/g-C3N4 may serve as an effective visible light-responsive catalyst for the degradation of organic dye pollutants, with promising applications.-
Keywords:
- MoO3 /
- BiOI /
- g-C3N4 /
- photocatalytic performance /
- double Z-scheme heterojunction photocatalyst
-
地球化学是研究地壳或地表中各类岩石、矿物、矿石及各种地质体中化学元素的组成、含量、分布及时空变化的学科,根据化学元素在地质体中含量的多少主要分为常量元素地球化学、微量元素地球化学、稀土元素地球化学等类型。通过对地球化学样品的研究,对农业发展、工业治理、环境监测、环境保护及地质资源勘查具有重要的指导意义。该类样品的测试,通常有测试批量大、元素多、工期紧等特点,因此开发协同、高效、准确的多元素测试方法显得尤为重要[1-7]。本文以地球化学中常见的硫、铁、铋、铅、锑、砷、汞等7种元素为研究目标,探索一种王水水浴消解,3种仪器分别测定的组合高效测试方法。
按现行行业标准规定,测定地球化学样品中硫[8]的方法主要X-荧光光谱法、红外碳硫仪法、燃烧碘量法等,本实验采用电感耦合等离子体发射光谱法测定,该方法具有测定步骤简单,线性范围宽,检测效率高的特点;铁[9]的测定主要有X-荧光光谱法、电感耦合等离子体发射光谱法,本文采用电感耦合等离子体发射光谱法测定铁。铋[10]的测定主要有原子荧光光谱法、电感耦合等离子体质谱法等,本文采用电感耦合等离子体质谱法测定,该方法具有测定方法检出限低,精密度和准确度高等特点;铅[11]的测定方法有X-荧光光谱法、电感耦合等离子体发射光谱法、电感耦合等离子体质谱法等,本文采用电感耦合等离子体质谱法测定;锑[12-13]的测定主要有电感耦合等离子体质谱法和原子荧光光谱法等,本文采用原子荧光光谱法测定;砷[14]的测定方法有电感耦合等离子体质谱法、原子荧光光谱法、电感耦合等离子体发射光谱法、X射线荧光光谱法等,本文采用原子荧光光谱法测定样品中的砷;汞[15]的测定方法有原子荧光光谱法、测汞仪法等,本文采用原子荧光光谱法测定。
本文结合地球化学样品测试的行业特点和7个待测元素的特殊属性,实现了共享使用同一种前处理方法底液,组合采用不同的仪器设备达到多种元素共同测定的目的。
1 实验
1.1 仪器及型号
实验采用的主要仪器设备如表 1所列。
表 1 实验仪器Table 1. Test Instruments
1.2 仪器工作参数及条件选择
电感耦合等离子体质谱(ICP-MS)已成为地球化学调查样品多元素分析中最重要的配套分析方法之一[16-18],具有其他分析技术不可比拟的优点,但质谱干扰仍是ICP-MS分析中不可忽视的问题[19-20],且在一定程度上限制了ICP-MS多元素同时分析的能力,甚至某些质谱干扰已成为痕量分析的严重障碍。由于地质样品的复杂性,干扰的校正是必不可少的,主要来自氧化物、多原子离子和同质异位素,其中多原子离子的干扰尤为严重,例如Ca、Cr、Ti等元素的氧化物对过渡元素的干扰,轻稀土元素的氧化物、氢氧化物对重稀土元素的干扰等,因此测定元素应尽可能选择不受干扰且丰度较高的同位素,本实验根据以往经验及仪器设备相关性能,选择208Pb,209Bi作为同位素;内标校正是通过在线加入与被测元素有相近的物理激发行为的元素,动态调控因仪器的漂移、溶液性质的变化等因素引起的对测量结果的影响加以校正,考虑到溶液中Rh的浓度很低,可以忽略不计,所以选择Rh作内标,同时用5%的逆王水清洗样品导入系统,可以减少记忆干扰对测定样品的影响。电感耦合等离子体质谱的工作参数设置见表 2。
表 2 电感耦合等离子体质谱仪工作参数Table 2. Working parameters of inductively coupled plasma mass spectrometer
电感耦合等离子体发射光谱法同样凭借其在多元素同时分析测试中的优异性能,被广泛应用于地质、环境、制药、食品等许多分析领域,但由于每个待测元素具有多条灵敏线,因此首先要考虑仪器提供的每个待测元素的信噪比和分析线的干扰情况[21-22],其次通过观察仪器软件自带的谱线相互干扰功能表进行考虑,最后考虑元素检出限、共存元素干扰、背景干扰和该元素线性范围[23]等因素。本文通过比较同一元素不同谱线的强度、峰形和有无谱线干扰等因素,选择强度大、峰形好和干扰小的2条谱线为分析线。同样需要注意的是,氩气吹扫时间也会对样品的准确测定有重要影响,因为波长在10~200 nm远紫外光能被光路中的空气(氧、氮、二氧化碳和水气)所吸收,波长越短的远紫外光越容易被空气吸蚀[24],本文选定的硫的分析谱线180. 67 nm,处于远紫外区,需要用氩气对光路和接口进行吹扫,通过试验及经验积累,氩气吹扫时间定在50 min以上,以确保同一浓度硫标准的谱线强度在1 h内变化小于1%。综上考虑,选择的电感耦合等离子体光谱仪分析线波长及背景校正模式见表 3,工作参数设置见表 4。
表 3 电感耦合等离子体发射光谱仪分析谱线的背景校正Table 3. Background correction of the spectral lines analyzed by inductively coupled plasma emission spectrometer 表 4 电感耦合等离子体质谱发射光谱仪工作参数Table 4. Operating parameters of icP-MS emission spectrometer
表 4 电感耦合等离子体质谱发射光谱仪工作参数Table 4. Operating parameters of icP-MS emission spectrometer
原子荧光光度计的使用目前已很成熟,本实验采用廊坊物探所XGY-1011A型原子荧光仪,具备低温点火原子化技术,除石英炉寿命较以往延长外,对测定的灵敏度也有极大的提高,同时记忆效应也明显降低。测定时根据多年工作经验,采用负高压250~270 V,载气流量700~900 mL /min,原子化温度为室温,炉温150~300 ℃。原子荧光光谱仪的工作参数设置见表 5。
表 5 原子荧光光谱仪工作参数(XGY-1011A)Table 5. Working parameters of atomic fluorescence spectrometer (XGY-1011A)
1.3 药品和试剂
盐酸,优级纯,江阴化学试剂厂有限公司;硝酸,优级纯,江阴化学试剂厂有限公司;硫脲,优级纯,天津科密欧化学试剂有限公司;抗坏血酸,优级纯,天津科密欧化学试剂有限公司;氯化亚锡,优级纯,天津科密欧化学试剂有限公司;硼氢化钾,优级纯,天津科密欧化学试剂有限公司;氢氧化钾,优级纯,天津科密欧化学试剂有限公司;铑标准溶液;实验用水为高纯水,电阻率为18.2 MΩ·cm。
1.4 样品和标准溶液
采用的实验样品为土壤成分分析国家一级标准物质,分别为GBW07453,GBW07454,GBW07455,GBW07456,GBW07457,GBW07385,均为中国地质科学院地球物理地球化学勘查研究院研制。本研究采用的标准溶液分别为:硫标准溶液(GSB04-1773-2004)、铅标准溶液(GSB04-1742-2004)、铋标准溶液(GSB04-1719-2004)、铁标准溶液(GSB04-1726-2004)、锑标准溶液(GSB04-1748-2004)、砷标准溶液(GSB04-1714-2004)、汞标准溶液(GSB04-1729-2004)。采用逐级稀释配制成标准工作溶液如表 6所列。
表 6 标准工作溶液表Table 6. Standard working solution table
1.5 分析步骤
称取待测样品(粒径小于74 μm)质量0.250 0 g(精确到0.1 mg),加入聚氯乙烯试管中,然后加入5 mL(1+1)王水与所述样品充分混合,盖上一层保险膜,用塑料板压住,置于95~100 ℃控温水域锅中加热溶解。待水沸腾后,保持沸水浴消解1 h,分解过程中不时晃动3~4次,消解结束后取出后试管,定容至25 mL,摇匀,静置4 h以上,得到待测试液。
分取5 mL待测试液至小烧杯中,定量加入1 mL(1+1)王水,再定量加入4 mL硫脲-抗坏血酸混合溶液,采用氢化物原子荧光法测定砷、锑;分取5 mL待测试液至50 mL容量瓶中,加水定容至50 mL,摇匀,采用电感耦合等离子体质谱法测定铋、铅;吸取待测试液2 mL,通过冷原子荧光法测定汞;剩余待测试液,采用电感耦合等离子体光谱法直接测定铁、硫。
2 结果与讨论
2.1 消解条件的选择
首先采用王水和水的比例分别为1∶1,1∶2,2∶1进行消解条件实验,实验对标准物质GBW07453和GBW07454进行测定,测定结果如表 7所列。
表 7 消解条件实验与测定结果Table 7. Results of digestion condition experiment and determination
从表 7的消解条件实验可以看出,王水和水的比例为1∶2时,样品消解不完全,当王水和水的比例为1∶1和2∶1时,样品消解较好,考虑到生产成本选择王水和水的比例为1∶1作为较优实验条件。
2.2 消解时间的选择
对标准物质GBW07453和GBW07454进行测定,考察了消解时间对测试结果的影响,结果如表 8所列。
表 8 消解时间条件实验与测定结果Table 8. Digestion time conditions and determination results
从表 8可以看出,标准物质元素含量测定值随着消解时间的增长而逐渐升高,消解时间1 h后,测定结果趋于稳定,说明样品消解完全,满足检测要求。因此,选择较优的消解时间为1 h。
2.3 方法的检出限
方法检出限参照《环境保护部环境监测分析方法标准制修订技术导则》(HJ 168—2010)进行,按照1.5分析步骤,对标准物质GBW07317进行测定,重复12次实验,取样量为0.25 g,定容为25 mL,将各测定结果换算为样品中的浓度,计算方法中各元素的检出限如表 9所列。
表 9 12次重复实验分析方法检出限Table 9. Detection limits of analysis method
从表 9可以看出,本文所测元素的检出限均满足或优于《多目标区域地球化学调查规范(1∶250000)》(DZ/T 0258/2014)要求。
2.4 方法的准确度和精密度分析
对标准物质GBW07453,GBW07454,GBW07455按照1.5实验方法进行测试,计算本方法的准确度和精密度,结果如表 10所列。
表 10 12次重复实验方法准确度和精密度Table 10. Method accuracy and precision
从表 10可以看出,方法中所测元素的准确度和精密度均满足或优于《多目标区域地球化学调查规范(1∶250000)》(DZ/T 0258—2014)要求。
2.5 加标回收率实验
取2份样品(1号样和2号样),按实验方法分别进行9次测定。同时,分别加入定量标准物质后再进行测定,见表 11。样品1和样品2中各元素的回收率分别为95%~104% 和96%~105%,相对标准偏差分别为0.13%~3.76%和0.55%~3.29%。该结果满足地质实验室质量管理规范的相关要求,精密度与回收率良好。
表 11 精密度与回收率实验Table 11. Experiment of precision and recovery
2.6 实验室外部控制样实验
按照实验方法对中国地质科学院地球物理地球化学勘查研究所的300件外部质量控制样品进行测定,考察本方法对实际样品测试的适用性。以150件为一个统计单元,共计2个单元,结果见表 12。
表 12 外部质量控制样品验证实验结果Table 12. Verification test results of external quality control samples
由表 12可知,本研究中硫、铁、铋、铅、锑、砷、汞等元素测定结果的合格率均大于98.0%,相关系数均大于0.900,F实测值均小于F单尾临界值,即全部满足合格率要求(按《多目标区域地球化学调查规范(1∶250000)》(DZ/T 0258—2014)要求需满足)。
3 结论
采用一次王水水浴溶解,3种仪器组合测定硫、铁、铋、铅、锑、砷、汞等7元素,与行业标准规定方法相比[25-30],有以下几个优点:
1)通过一次王水水浴消解获取底液,组合测定地球化学样品中硫、铁、铋、铅、锑、砷、汞等7种元素,相比较行业标准方法更加高效、便捷,且测试的检出限、准确度和精密度均满足或优于规范要求;
2)在当前生态环保优先的形势下,本方法与行业标准相比,能显著降低试剂使用,减少环境危害,且有效降低测试成本;
3)针对的区域地球化学类的样品测试,样品量大,且测试元素繁杂,测试工期要求紧,本方法能够高效、协同实现多种元素同步测试,尤其是针对地质实验测试大批量操作具有较好的推动作用,值得广大地质实验室推广应用。
-
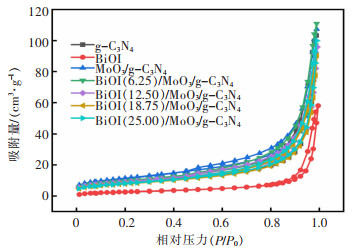
表 1 样品的比表面积,孔容和孔径
Table 1 Specific surface area, pore volume and pore size of the sample

表 2 样品的吸收边和带隙能
Table 2 The absorption edge and bandgap energy of the sample

表 3 样品的带隙能(Eg)、导带能(ECB)和价带能(EVB)
Table 3 Sample bandgap (Eg), conduction belt (ECB) and price band (EVB)

-
[1] LEWIS N S. Research opportunities to advance solar energy utilization[J]. Science, 2016, 351(6271): 1920. doi: 10.1126/science.aad1920
[2] GONG J L, CAN L, WASIELEWSKE M R. Advances in solar energy conversion[J]. Chemical Society Reviews, 2019, 48(7): 1862-1864. doi: 10.1039/C9CS90020A
[3] FUJISHIMA A, HONDA K. Electrochemical photolysis of water at a semiconductor electrode[J]. Nature, 1972, 238(5358): 37-38. doi: 10.1038/238037a0
[4] WANG Q, DOMEN K. Particulate photocatalysts for light-driven water splitting: mechanisms, challenges, and design strategies[J]. Chemical Reviews, 2019, 120(2): 919-985.
[5] DENG Y, ZHAO R Z. Advanced oxidation processes (AOPs) in wastewater treatment[J]. Current Pollution Reports, 2015, 1(3): 167-176. doi: 10.1007/s40726-015-0015-z
[6] 马小帅, 陈范云, 张萌迪, 等. g-C3N4基光催化剂的制备和应用[J]. 有色金属科学与工程, 2018, 9(3): 42-52. doi: 10.13264/j.cnki.ysjskx.2018.03.008 [7] GARCIA B B, LOURINHO G, ROMANO P, et al. Photocatalytic degradation of swine wastewater on aqueous TiO2 suspensions: optimization and modeling via box-behnken design[J]. Heliyon, 2020, 6(1): e03293. doi: 10.1016/j.heliyon.2020.e03293
[8] ONG W J, TAN L L, NG Y H, et al. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: are we a step closer to Achieving Sustainability?[J]. Chemical Reviews, 2016, 116(12): 7159-7329. doi: 10.1021/acs.chemrev.6b00075
[9] LIU G M, DONG G H, ZENG Y B, et al. The photocatalytic performance and active sites of g-C3N4 effected by the coordination doping of Fe(Ⅲ)[J]. Chinese Journal of Catalysis, 2020, 41(10): 1564-1572. doi: 10.1016/S1872-2067(19)63518-7
[10] FU Y H, LI Z J, LIU Q Q, et al. Construction of carbon nitride and MoS2 quantum dot 2D/0D hybrid photocatalyst: Direct Z-scheme mechanism for improved photocatalytic activity[J]. Chinese Journal of Catalysis, 2017, 38(12): 2160-2170. doi: 10.1016/S1872-2067(17)62911-5
[11] 叶仕雄, 舒庆. 硝酸活化三聚氰胺前驱体对g-C3N4结构和可见光催化性能的影响[J]. 无机化学学报, 2020, 36(1): 1-10. https://www.cnki.com.cn/Article/CJFDTOTAL-WJHX202001001.htm [12] LI Y, ZHANG D N, FAN J J, et al. Highly crystalline carbon nitride hollow spheres with enhanced photocatalytic performance[J]. Chinese Journal of Catalysis, 2021, 42(4): 627-636. doi: 10.1016/S1872-2067(20)63684-1
[13] VIGNESH S, MUPPUDATHI A L, SUNDAR J K. Multifunctional performance of g-C3N4-BiFeO3-Cu2O hybrid nanocomposites for magnetic separable photocatalytic and antibacterial activity[J]. Journal of Materials Science: Materials in Electronics, 2018, 29(13): 10784-10801. doi: 10.1007/s10854-018-9144-7
[14] SUN Q, LV K, ZHANG Z H, et al. Effect of contact interface between TiO2 and g-C3N4 on the photoreactivity of g-C3N4/TiO2 photocatalyst: (001) vs (101) facets of TiO2[J]. Applied Catalysis B: Environmental, 2015, 164: 420-427. doi: 10.1016/j.apcatb.2014.09.043
[15] MUNIANDY L, ADAM F, MOHAMED A R, et al. Cu2+ coordinated graphitic carbon nitride (Cu-g-C3N4) nanosheets from melamine for the liquid phase hydroxylation of benzene and VOCs[J]. Applied Surface Science, 2017, 398: 43-45. doi: 10.1016/j.apsusc.2016.11.103
[16] LIU C, FENG Y, HAN Z T, et al. Z-scheme N-doped K4Nb6O17/g-C3N4 heterojunction with superior visible-light-driven photocatalytic activity for organic pollutant removal and hydrogen production[J]. Chinese Journal of Catalysis, 2021, 42(1): 164-174. doi: 10.1016/S1872-2067(20)63608-7
[17] HE F, LUO L, WANG Z X, et al. Supramolecule self-assembly synthesis of condensed C-TA/g-C3N4 composites for promoting charge transfer and photocatalytic H2 evolution[J]. Applied Surface Science, 2020, 504: 144354. doi: 10.1016/j.apsusc.2019.144354
[18] FENG Z, ZENG L, ZHANG Q L, et al. In situ preparation of g-C3N4/Bi4O5I2 complex and its elevated photoactivity in Methyl Orange degradation under visible light[J]. Journal of Environmental Sciences, 2020, 87: 149-162. doi: 10.1016/j.jes.2019.05.032
[19] 张川群, 周勤, 徐冲, 等. Bi2MoO6的形貌调控及其应用研究进展[J]. 有色金属科学与工程, 2021, 12(2): 56-65. doi: 10.13264/j.cnki.ysjskx.2021.02.008 [20] XUE M R, WANG X K, LI X Q, et al. C3N4 nanosheets loaded with the CuWO4 activated NiS co-catalyst: A stable noble metal-free photocatalyst with dramatic photocatalytic activity for H2 generation and high salinity tolerant[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2021, 405: 112919. doi: 10.1016/j.jphotochem.2020.112919
[21] CHEN J, LIU T, ZHANG H, et al. One-pot preparation of double S-scheme Bi2S3/MoO3/C3N4 heterojunctions with enhanced photocatalytic activity originated from the effective charge pairs partition and migration[J]. Applied Surface Science, 2020, 527: 146788. doi: 10.1016/j.apsusc.2020.146788
[22] DI L J, YANG H, XIAN T, et al. Photocatalytic and photo-Fenton catalytic degradation activities of Z-scheme Ag2S/BiFeO3 heterojunction composites under visible-light irradiation[J]. Nanomaterials, 2019, 9(3): 399. doi: 10.3390/nano9030399
[23] LI X, SHEN D, LIU C, et al. Fabricated rGO-modified Ag2S nanoparticles/g-C3N4 nanosheets photocatalyst for enhancing photocatalytic activity[J]. Journal of Colloid and Interface Science, 2019, 554: 468-478. doi: 10.1016/j.jcis.2019.07.027
[24] ZENG L, ZHE F, WANG Y, et al. Preparation of interstitial carbon doped BiOI for enhanced performance in photocatalytic nitrogen fixation and methyl orange degradation[J]. Journal of Colloid and Interface Science, 2019, 539: 563-574. doi: 10.1016/j.jcis.2018.12.101
[25] TAN Y G, SHU Z, ZHOU J, et al. One-step synthesis of nanostructured g-C3N4/TiO2 composite for highly enhanced visible-light photocatalytic H2 evolution[J]. Applied Catalysis B: Environmental, 2018, 230: 260-268. doi: 10.1016/j.apcatb.2018.02.056
[26] LI Y, HE R, HAN P, et al. A new concept: volume photocatalysis for efficient H2 generation_using low polymeric carbon nitride as an example[J]. Applied Catalysis B: Environmental, 2020, 279: 119379. doi: 10.1016/j.apcatb.2020.119379
[27] JUNG H, PHAM T T, SHIN E W. Effect of g-C3N4 precursors on the morphological structures of g-C3N4/ZnO composite photocatalysts[J]. Journal of Alloys and Compounds, 2019, 788: 1084-1092. doi: 10.1016/j.jallcom.2019.03.006
[28] 张彩霞, 霍彦廷, 邹来禧, 等. 碳纳米球复合g-C3N4提升光催化降解酸性橙Ⅱ性能[J]. 复合材料学报, 2021, 38(11): 3877-3887. https://www.cnki.com.cn/Article/CJFDTOTAL-FUHE202111029.htm [29] PANG M L, HU J Y, ZENG H C. Synthesis, morphological control, and antibacterial properties of hollow/solid Ag2S/Ag heterodimers[J]. Journal of the American Chemical Society, 2010, 132(31): 10771-10785. doi: 10.1021/ja102105q
[30] 李笑笑, 杨凯, 曾德彬, 等. 微波水热法制备棒状BiPO4催化剂及其光催化性能研究[J]. 有色金属科学与工程, 2019, 10(4): 78-84. doi: 10.13264/j.cnki.ysjskx.2019.04.013 [31] SHEN T T, LANG D, CHENG F Y, et al. Ternary reduced graphene oxide/g-C3N4/Ag-AgCl nanoco mposites for controlled visible-light photocatalytic selectivity[J]. Chemistry Select, 2016, 1(5): 1006-1015.
[32] HU S Z, MA L, XIE Y, et al. Hydrothermal synthesis of oxygen functionalized S-P codoped g-C3N4 nanorods with outstanding visible light activity under anoxic conditions[J]. Dalton Transactions, 2015, 44(48): 20889-20897. doi: 10.1039/C5DT04035C
[33] 杜瑞安, 马小帅, 张萌迪, 等. 多壁碳纳米管/TiO2复合材料的合成及其光催化性能[J]. 有色金属科学与工程, 2019, 10(5), 75-84. doi: 10.13264/j.cnki.ysjskx.2019.05.012 [34] LI Y P, WU S L, HUANG L Y, et al. Synthesis of carbon-doped g-C3N4 composites with enha nced visible-light photocatalytic activity[J]. Materials Letters, 2014, 137: 281-284. doi: 10.1016/j.matlet.2014.08.142
[35] SHENG Y Q, WEI Z, MIAO H, et al. Enhanced organic pollutant photodegradation via adsorpt -ion/photocatalysis synergy using a 3D g-C3N4/TiO2 free-separation photocatalyst[J]. Chemical Engineering Journal, 2019, 370: 287-294. doi: 10.1016/j.cej.2019.03.197
[36] ZHAO X L, PAN D L, CHEN X F, et al. g-C3N4 photoanode for photoelectrocatalytic synergistic pollutant degradation and hydrogen evolution[J]. Applied Surface Science, 2019, 467: 658-665.
[37] HUO Y, ZHANG J F, DAI K, et al. All-solid-state artificial Z-scheme porous g-C3N4/Sn2S3-DETA heterostructure photocatalyst with enhanced performance in photocatalytic CO2 reduction[J]. Applied Catalysis B: Environmental, 2019, 241: 528-538. doi: 10.1016/j.apcatb.2018.09.073
[38] FU J W, XU Q L, LOW J X, et al. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst[J]. Applied Catalysis B: Environmental, 2019, 243: 556-565. doi: 10.1016/j.apcatb.2018.11.011
[39] MURUGESAN P, NARAYANAN S, MANICKAM M, et al. A direct Z-scheme plasmonic AgCl@ g-C3N4 heterojunction photocatalyst with superior visible light CO2 reduction in aqueous medium[J]. Applied Surface Science, 2018, 450: 516-526. doi: 10.1016/j.apsusc.2018.04.111
[40] ZHU A S, ZHAO Q D, LI X Y, et al. BiFeO3/TiO2 nanotube arrays composite electrode: construction, characterization, and enhanced photoelectrochemical properties[J]. ACS Applied Materials & Interfaces, 2014, 6(1): 671-679.
[41] BAGVAND N, SHARIFNIA S, KARAMIAN E. A visible-light-active BiFeO3/ZnS nanocomposite for photocatalytic conversion of greenhouse gases[J]. Korean Journal of Chemical Engineering, 2018, 35(8): 1735-1740. doi: 10.1007/s11814-018-0083-z
[42] SAMRAN B, TONNONCHIANG S, CHAIWICHIAN S. BiFeO3/BiVO4 nanocomposite photocatalysts with highly enhanced photocatalytic activity for rhodamine B degradation under visible light irradiation[J]. Physica B: Condensed Matter, 2019, 561: 23-28. doi: 10.1016/j.physb.2019.02.049
[43] LIAO G Z, CHEN S, QUAN X, et al. Graphene oxide modified g-C3N4 hybrid with enhanced pho photocatalytic capability under visible light irradiation[J]. Journal of Materials Chemistry, 2012, 22(6): 2721-2726. doi: 10.1039/C1JM13490F
[44] LI J, YU X, ZHU Y, et al. 3D-2D-3D BiOI/porous g-C3N4graphene hydrogel composite photocatalyst with synergy of adsorption-photocatalysis in static and flow systems[J]. Journal of Alloys and Compounds, 2021, 850: 156778. doi: 10.1016/j.jallcom.2020.156778
[45] JIANG T, WANG K, GUO T, et al. Fabrication of Z-scheme MoO3/Bi2O4 heterojunction photocatalyst with enhanced photocatalytic performance under visible light irradiation[J]. Chinese Journal of Catalysis, 2020, 41(1): 161-169. doi: 10.1016/S1872-2067(19)63391-7
[46] FAN T, CHEN C C, TANG Z H, et al. Synthesis and characterization of g-C3N4/BiFeO3 composites with an enhanced visible light photocatalytic activity[J]. Materials Science in Semiconductor Processing, 2015, 40: 439-445. doi: 10.1016/j.mssp.2015.06.054
-
期刊类型引用(7)
1. 梁俊生. 原子荧光法同时测定混合铅锌精矿中的砷和锑. 韶关学院学报. 2024(12): 18-24 .  百度学术
百度学术
2. 朱国政,廖智,林常青,张国斐,刁琪琪. 原子荧光光谱法的断续流动进样体积差异对比. 江西化工. 2023(01): 53-55+59 .  百度学术
百度学术
3. 王茜,徐崇颖,杨嘉晖,陈超,祁春景,王旭东. 利用控制图法评定原子荧光光谱法测定土壤中总砷含量的测量不确定度. 冶金分析. 2023(02): 31-38 .  百度学术
百度学术
4. 熊玉宝,郝宏艳,邹婷婷. 电感耦合等离子体发射光谱法测定多目标区域地球化学样品中硫的不确定度评定. 造纸装备及材料. 2022(03): 82-84 .  百度学术
百度学术
5. 何绒. 原子荧光光谱法测定铅锌尾矿中砷、锑和铋含量. 世界有色金属. 2022(06): 162-164 .  百度学术
百度学术
6. 王景凤,赖春华,孙康,宋学文,孔会民,王茂盛,隆英兰. 基于电感耦合等离子技术测定高纯氧化镁产品中酸溶硅含量. 有色金属科学与工程. 2022(04): 135-140 .  本站查看
本站查看
7. 宋玉冰. 特殊地质样品中钼同位素分析的化学前处理方法分析. 世界有色金属. 2021(18): 135-136 .  百度学术
百度学术
其他类型引用(0)



 下载:
下载: