Research progress in fabrication and application of S-scheme heterojunction photocatalysts
-
摘要: 近年来,研究者提出了一种新的S-型异质结光催化剂。S-型异质结光催化剂在光生电子空穴对的有效分离、无用电子空穴的复合、载流子的有效分离、保留强氧化-还原活性位点等方面有着显著的优点。综述了近年来,S-型异质结光催化剂的反应机理、制备方法,以及在水分解制氢、CO2还原、污染物的降解、灭菌等应用方面的研究进展,提出了S-型光催化体系的发展前景和面临的挑战。Abstract: In recent years, researchers have proposed a new S-scheme heterojunction photocatalyst, which has distinct advantages in the effective separation of photogenerated electron-hole pairs, recombination of useless electrons and holes, high separation efficiency of charge carriers, and retention of strong oxidation reduction active sites. In this paper, the reaction mechanism and preparation method of S-scheme heterojunction photocatalyst and the research progress of its application in water decomposition to produce hydrogen, CO2 reduction, and the degradation and sterilization of pollutants have been summarized. Finally, prospects and challenges for the development of S-scheme photocatalytic systems are presented.
-
炼钢渣系是以石灰为主的CaO-FeO-SiO2渣系,该渣系具有熔点高、石灰溶解速率低的缺点,是限制其高效冶炼的关键因素[1-2]。为降低入转炉的磷负荷,缩短冶炼周期,工业上采用铁水预处理操作将脱磷工序提前,此时预处理温度较低,具有较好的脱磷热力学条件。但是,此时渣流动性差,动力学条件不佳,需要合适的助熔剂快速化渣,而现有的含氟熔剂污染环境,因此,新型无氟熔剂的开发势在必行[3-4]。有研究发现含有Na2O、A12O3、TiO2组元的赤泥基熔剂对传统的炼钢渣系具有显著的助熔作用,然而其对石灰溶解的影响机理依然不清楚[5-11]。
SHIRO等[12]在研究石灰溶解的影响因素时发现,Al2O3的存在使得CaO-Al2O3-FexOy通量促进了石灰的溶解,它可以作为CaO-CaF2-FexOy通量的替代品。MUKAWA等[13-14]分别确定了Na2O-CaO和CaO-Al2O3通量的最佳Na2O和Al2O3含量。SHIMODA等[15]提出,CaO-Al2O3-TiO2通量由于其低熔点和黏度,显著促进了熔融铁的脱硫。此外,LEE等[16]开发了一种涂覆二钙铁氧体(2CaO·FeO)的新型生石灰(氧化钙),其具有较高的抗水化性能,可快速溶解于BOF和EAF炼钢渣中。HAMANO等[17]发现,CaF2和CaCl2的加入导致矿渣中石灰的溶解率较高[18-20]。
因此,本文研究了Al2O3/TiO2/Na2O对石灰在炼钢渣系中溶解速率的影响并探究其机理,为赤泥基化渣剂在炼钢中的规模化应用提供理论支撑。
1 实验部分
1.1 实验原料及设备
本研究使用的炉渣由分析纯试剂合成。实验中将Na2O和P2O5分别替换为无水硅酸钠和磷酸钙。所用实验原料化学成分见表1。试样0为空白炉渣CaO- SiO2-FeO-MgO-P2O5;样品A、T和N分别由含10% Al2O3、TiO2和Na2O的炉渣组成;样品AT为含5% Al2O3和5%TiO2的炉渣;样品AN为含5% Al2O3和5% Na2O的炉渣。样品ATN为含10% Al2O3、TiO2和Na2O的混合物Al2O3∶TiO2∶Na2O = 15∶4∶3(该比值代表某厂添加赤泥后的炉渣成分)。由于石灰溶解主要发生在转炉炼钢前期,所有炉渣初始二元碱度均设为1。
表 1 实验原料化学成分Table 1. Experimental raw materials样品编号 CaO SiO2 FeO Al2O3 TiO2 Na2O MgO P2O5 0 36 36 20 0 0 0 5 3 A1 31 31 20 10 0 0 5 3 T1 31 31 20 0 10 0 5 3 N1 31 31 20 0 0 10 5 3 ATN 31 31 20 6.8 1.8 1.4 5 3 AT 31 31 20 5 5 0 5 3 AN 31 31 20 5 0 5 5 3 为制得实验需要的活性石灰圆柱试样,采用12 g分析纯CaO(国药)作原料。首先破碎成粉,再用水和有机黏结剂粘结,通过压样机压制成带内孔的石灰圆柱(d外=20 mm,d内=7 mm,h=20 mm,P=30 MPa),然后在电阻炉内1 200 ℃温度下干燥120 min,得到活性石灰柱,其密度在1.46~1.50 g/cm3之间。干燥后的石灰柱的尺寸为:d外= (20 ± 1) mm,d内= (7 ± 1) mm,h= (20 ± 1) mm。
石灰柱的顶面及底面均有垫有钼垫圈。采用图1所示的旋转圆盘装置进行溶出实验。本实验采用立式硅钼炉作为加热装置,实验前对热电偶进行了标定。本实验采用的保护气体为高纯氩气。
首先将170 g渣样放入氧化镁坩埚(d内=60 mm,d外=70 mm,h=100 mm)中。根据图2中的温度曲线,利用管式炉将炉渣加热至1 400 ℃。待渣样熔化并保持温度恒定在目标值后,将石灰柱缓慢放入渣中。立即启动电机,转速为150 r/min。根据石灰柱完全溶解所需的时间,每组实验分别进行4次,即3、5、10 min和15(20) min,对于20 min完全溶解的实验,采用15 min时得到的数据。
实验结束后,立即将石灰柱从渣中抬出,并将黏附在表面的渣高速旋转甩落。此后,将试样从炉口取出,在高温下用数显游标卡尺直接测量不同部位的直径。采用水平平板拍照,以直径平均值计算试样直径减小值。并取终渣样品进行X射线荧光分析,计算渣中石灰溶出量。石灰溶解后,将石灰柱装入环氧树脂中抛光。然后对其进行喷金处理,采用扫描电镜(SEM)观察石灰/矿渣界面,并采用能谱仪(EDS)进行化学分析。
1.2 石灰溶解速率的计算方法
在石灰柱上下两侧放置钼垫圈,防止石灰柱与矿渣接触,溶解物质集中在侧面。石灰溶解率(X)的定义为:
% (1) 式(1)中:r0和rc分别为石灰柱的初始半径和剩余半径。
通过实验中石灰柱的单位时间半径的变化测定石灰在渣中的溶解速率,溶解速率为:
(2) 式(2)中:vr为石灰的溶解速度的数值,单位m/ s ;V为石灰圆柱的体积的数值,单位m3 ; S为石灰圆柱的侧面积的数值,单位m2 ; r 为石灰圆柱的半径的数值,单位m 。
2 结果与讨论
2.1 Al2O3/TiO2/Na2O对石灰溶解速率的影响
图3所示为添加不同炉渣对石灰溶解的影响。结果表明,Al2O3、TiO2和Na2O的加入促进了石灰的溶解。石灰在样品N(在5 min内观察到50%的石灰溶解,在10 min内达到90%的溶解)中溶解最快。样品T在15 min后溶解100%的石灰,比样品A溶解石灰更快。不含添加剂的样品0对石灰的溶解速率最慢,20 min时仅有52%的石灰柱溶解。添加5% Na2O的AN样品中石灰的溶出明显快于AT样品。此外,样品ATN中石灰的溶解速度快于样品AN和AT。
图3表明,除含Na2O的样品N、AN和ATN外,石灰在其他样品中的溶解过程可分为2个不同阶段:5 min前缓慢溶解和5 min后快速溶解。石灰在渣样中溶解5、10 min的溶出率如图4所示。第一阶段由于附近的炉渣与石灰柱接触后冷却,降低了其流动性,导致石灰缓慢溶解。对于含Na2O的样品,没有观察到明显的第一阶段,是因为这些样品可以显著降低熔渣的熔点。第二阶段发生在前5 min之后,石灰溶解速率随着时间的延长呈线性变化。
根据表2中第二溶解阶段的线性拟合计算了石灰的溶解速率,拟合度R2均在0.9以上。
表 2 石灰溶解率Table 2. Lime dissolution rate样品 0 A T N ATN AN AT 第二溶解阶段 2.77 3.93 7.69 9.75 8.08 6.65 5.83 为了定量评价添加Al2O3/TiO2/Na2O对CaO-SiO2-FeO-MgO-P2O5熔渣中石灰溶解的促进效果。定义A为促进系数,即单位Al2O3/TiO2/Na2O含量对原渣系中石灰溶出率的促进作用。计算公式如式(3)所示:
(3) 式(3)中:
0和 分别为添加Al2O3/TiO2/Na2O前后石灰的溶出速率;X为添加Al2O3/TiO2/Na2O的质量百分比。 从表3列出的促进系数来看,Na2O对炉渣中石灰溶解的促进作用大于Al2O3。因此,仅添加少量的Na2O即可大幅提高石灰的溶出量,得到的促进系数取值可用于实际生产。
表 3 不同添加剂对石灰溶解速率的促进系数Table 3. Promotion coefficients of different additives on lime dissolution rate添加剂 Al2O3 TiO2 Na2O 促进系数(A) 1.162 × 10-6 4.917 × 10-6 6.98 × 10-6 2.2 Al2O3/TiO2/Na2O对石灰溶解过程中微观结构的影响
图5(a)—图5(e)分别为试样0、A、T、N、ATN在石灰中溶解10 min后,石灰-矿渣界面微观形貌和物相组成的SEM像。基于SEM分析,在石灰溶解过程中,除了第1相初始渣和第5相石灰外,界面附近新生成了4种类型的相:第1相为初始渣;第2相(C2S)为含有Ca2SiO4等硅酸盐相;第3相(MF)为边界层,Fe、Mg含量较高;第4相(CF)为侵入性矿渣,是侵入石灰孔隙的矿渣,是由铁酸钙等化合物组成的高铁相;第5相为残余固体石灰;第6相为含Ti相,含有CaTiO3和CaSiTiO5等化合物。
图6所示为Factsage软件7.0计算的石灰溶解过程中的理论生成的物相。样本0包含相(1)—相(5)。从图5(b)可以看出,加入样品A渣后没有新相生成,观察到边界上没有MF相。这与Factsage软件(图6(b))的预测一致。由于结构和溶解速率变化的综合作用,Al主要存在于熔渣和MF边界层中。.
图5(c)表明,添加10% TiO2后,样品T中形成了包含CaTiO3和CaSiTiO5相的物相。随着溶解速率的变化,含Ti相消耗了渣边界附近的C2S层和CaO,加速了传质,加快了第一阶段C2S层在石灰溶解中的突破速率和溶解速率。TANG等[21]在FeOx-SiO2-V2O3-TiO2渣系中也报道了渣中含Ti相,如CaTiO3和CaSiTiO5。与样品A中的Al不同,样品T的边界层或石灰间隙中没有检测到Ti。
图5(d)所示为样品N的显微结构和物相组成的SEM像,与样品0相比,C2S层没有出现在渣-石灰界面处,说明10% Na2O的加入阻碍了C2S层的形成,提高了CaO在石灰柱中的传质系数(k)。这也解释了在样品N和AN中没有观察到石灰溶解第一阶段的原因。
图5(e)给出了加入10% ATN后炉渣的物相变化和石灰的溶解情况,没有观察到明显的新相。
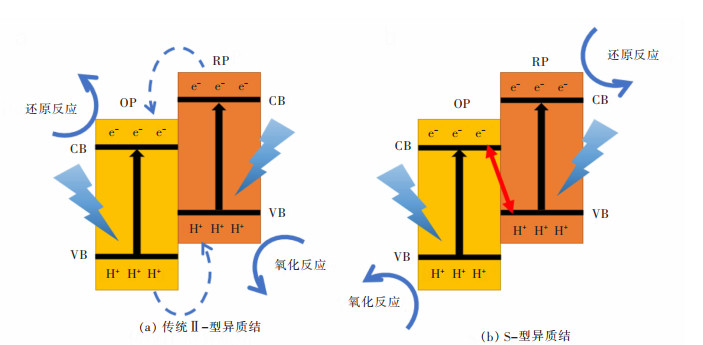
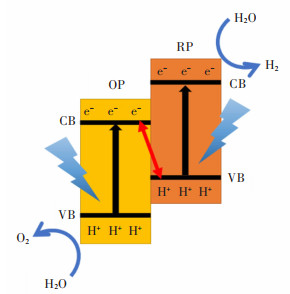
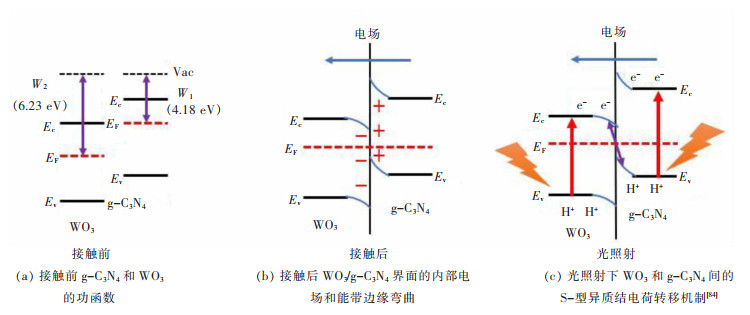
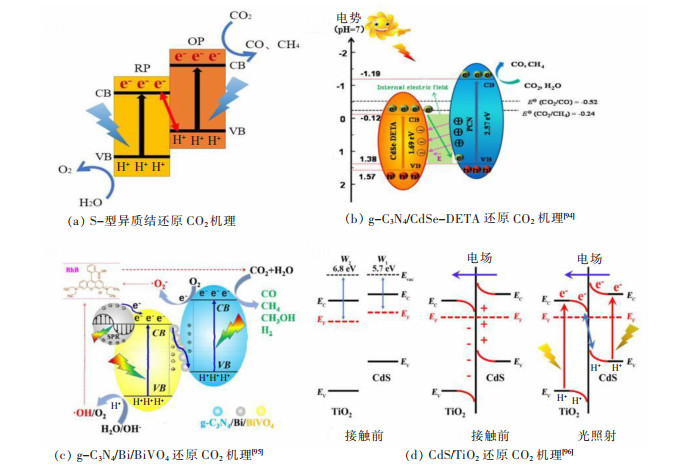
2.3 Al2O3/TiO2/Na2O对石灰溶解机理的影响
如图7所示为传统转炉渣中石灰的溶解过程,包括3个基本步骤:①在渣与石灰的界面,石灰从固相分解为液相的反应。②石灰通过反应产物层(C2S层)。③石灰通过边界层扩散到矿渣中。而加入赤泥基化渣剂后C2S层消失,因此石灰溶解减少为2个步骤:①在渣与石灰的界面,石灰从固相分解为液相的反应。②石灰通过边界层扩散到矿渣中。
因此改变CaO加入量后对石灰溶解速率的影响主要从步骤①和步骤②考虑。石灰从固相到液相的相变过程存在式(4)—式(6)反应。
CaO(s)CaO(l) (4) K =C (CaO(l))/C (CaO(s))(5) ln K =ln(C (CaO(l))-C (CaO(s))=ln(Δω (CaO))(6) C(CaO(s))为分解的CaO表面与熔渣界面处CaO的体积浓度,可由液相线与连接熔渣成分点和CaO顶点的直线的交点确定;C(CaO(l))为熔渣中(CaO)的体积浓度。计算得到Δω(CaO)=C(CaO(l))- C(CaO(s)。
图8和图9所示分别为使用Factsage计算的CaO添加量对熔渣黏度和Δω(CaO)的影响。Δω(CaO)大小对应着步骤①反应的快慢,当添加10% Al2O3时,熔渣黏度从1.208 dPa·s增加到2.325 dPa·s,使得CaO在熔渣中的扩散变慢,降低石灰溶解速率;而另一方面,Δω(CaO)也同样增加,使得在边界层上的石灰分解反应加快,步骤①和步骤②的一增一减最终导致石灰溶解速率仅略有增加。
当添加10% TiO2时,熔渣黏度从1.208 dPa·s降低到0.683 5 dPa·s,使得CaO在熔渣中的扩散加速,促进了石灰溶解速率;而另一方面,Δω(CaO)也同样增加,使得在边界层上的石灰分解反应加快,导致石灰溶解速率明显加快。
当添加10% Na2O,熔渣黏度从1.208 dPa·s降低到0.758 dPa·s,使得CaO在熔渣中的扩散加速,石灰加快溶解;而另一方面,Δω(CaO)也同样增加,使得在边界层上的石灰分解反应加快,2个步骤均加速,导致石灰溶解速率明显加快。
当添加10% ATN时,熔渣黏度从1.208 dPa·s略微增加到1.44 dPa·s,对CaO在熔渣中的扩散影响较小;而另一方面,Δω(CaO)明显增加,使得在边界层上的石灰溶解反应加快,最终导致石灰溶解速率明显加快。
其中Na2O对加速石灰溶解有促进作用,但过高的Na2O含量会造成耐火材料的腐蚀,对转炉的连续运行产生不利影响。目前,使用赤泥作为熔剂后渣中Na2O含量低于1.5%,因此使用有限的赤泥不会加剧耐火材料的侵蚀[22]。
3 结 论
1)在CaO-SiO2-FeO-MgO-P2O5当矿渣中加入10% Al2O3/TiO2/Na2O时, Al2O3、TiO2、Na2O在强制对流下对石灰的溶解均有促进作用。添加剂对矿渣的影响由小到大为:Al2O3<TiO2<Na2O,促进系数分别为1.162×10-6、4.917×10-6、6.98×10-6 cm/s。
2)Al2O3的添加一方面使得CaO在熔渣中的扩散变慢,另一方面使得边界层上的石灰分解反应加快,最终导致石灰溶解速率略有增加。TiO2和Na2O的添加使得CaO在熔渣中的扩散和边界层上的石灰分解反应均加快,导致石灰溶解速率明显加快。
3)当Al2O3、TiO2、Na2O按照不同比例组成时,得到的ATN赤泥基熔剂一方面对CaO在熔渣中的扩散影响较小,另一方面大幅加快了边界层上的石灰分解反应,能获得较快的石灰溶解速率,该熔剂可以作为炼钢熔剂。
-
表 1 S-型异质结构建方法
Table 1 Construction method of S-scheme heterojunction

表 2 用于光解水产氢的S-型异质结光催化剂
Table 2 Hydrogen production over S-scheme heterojunction photocatalysts

表 4 用于CO2还原的S-型异质结光催化剂
Table 4 Reduction of CO2 over S-scheme heterojunction photocatalysts

表 5 用于降解污染物的S-型异质结光催化剂
Table 5 Degradation of pollutants over S-scheme heterojunction photocatalysts

-
[1] BAHRUDIN N N, NAWI M A. Immobilized titanium dioxide/powdered activated carbon system for the photocatalytic adsorptive removal of phenol[J]. Korean Journal of Chemical Engineering, 2018, 35(7): 1532-1541. doi: 10.1007/s11814-018-0062-4
[2] CHEN X, ZHANG J, ZENG J, et al. Novel 3D/2D heterojunction photocatalysts constructed by three-dimensional In2S3 dandelions and ultrathin hexagonal SnS2 nanosheets with excellent photocatalytic and photoelectrochemical activities[J]. Applied Surface Science, 2019, 463: 693-703. doi: 10.1016/j.apsusc.2018.09.013
[3] YANG J, ZHU X, MO Z, et al. A multidimensional In2S3-CuInS2 heterostructure for photocatalytic carbon dioxide reduction[J]. Inorganic Chemistry Frontiers, 2018, 5(12): 3163-3169. doi: 10.1039/C8QI00924D
[4] YUAN X, JIANG L, LIANG J, et al. In-situ synthesis of 3D microsphere-like In2S3/InVO4 heterojunction with efficient photocatalytic activity for tetracycline degradation under visible light irradiation[J]. Chemical Engineering Journal, 2019, 356: 371-381. doi: 10.1016/j.cej.2018.09.079
[5] XIAO T, TANG Z, YANG Y, et al. In situ construction of hierarchical WO3/g-C3N4 composite hollow microspheres as a Z-scheme photocatalyst for the degradation of antibiotics[J]. Applied Catalysis B: Environmental, 2018, 220: 417-428. doi: 10.1016/j.apcatb.2017.08.070
[6] JIANG R, WU D, LU G, et al. Modified 2D-2D ZnIn2S4/BiOCl van der Waals heterojunctions with CQDs: accelerated charge transfer and enhanced photocatalytic activity under vis-and NIR-light[J]. Chemosphere, 2019, 227: 82-92. doi: 10.1016/j.chemosphere.2019.04.038
[7] ZHAO S, HU F, LI J. Hierarchical core-shell Al2O3@ Pd-CoAlO microspheres for low-temperature toluene combustion[J]. Acs Catalysis, 2016, 6(6): 3433-3441. doi: 10.1021/acscatal.6b00144
[8] 娄向东, 魏崇, 李炳鑫, 等. C@ ZnFe2O4/Ag3PO4复合材料的可见光催化性能研究[J]. 河南师范大学学报(自然科学版), 2019, 47(5): 78-84. https://www.cnki.com.cn/Article/CJFDTOTAL-HNSX201905013.htm [9] 王颖, 杨传玺, 王小宁, 等. 二维光催化材料研究进展[J]. 有色金属科学与工程, 2021, 12(2): 30-42. doi: 10.13264/j.cnki.ysjskx.2021.02.005 [10] ZHAO Y, LIANG X, SHI H, et al. Photocatalytic activity enhanced by synergistic effects of nano-silver and ZnSe quantum dots co-loaded with bulk g-C3N4 for Ceftriaxone sodium degradation in aquatic environment[J]. Chemical Engineering Journal, 2018, 353: 56-68. doi: 10.1016/j.cej.2018.07.109
[11] XU Y, GE F, CHEN Z, et al. One-step synthesis of Fe-doped surface-alkalinized g-C3N4 and their improved visible-light photocatalytic performance[J]. Applied Surface Science, 2019, 469: 739-746. doi: 10.1016/j.apsusc.2018.11.062
[12] XU Y, LIU J, XIE M, et al. Construction of novel CNT/LaVO4 nanostructures for efficient antibiotic photodegradation[J]. Chemical Engineering Journal, 2019, 357: 487-497. doi: 10.1016/j.cej.2018.09.098
[13] SHARMA G, KUMAR A, NAUSHAD M, et al. Photoremediation of toxic dye from aqueous environment using monometallic and bimetallic quantum dots based nanocomposites[J]. Journal of Cleaner Production, 2018, 172: 2919-2930. doi: 10.1016/j.jclepro.2017.11.122
[14] YE Z, LI J, ZHOU M, et al. Well-dispersed nebula-like ZnO/CeO2@ HNTs heterostructure for efficient photocatalytic degradation of tetracycline[J]. Chemical Engineering Journal, 2016, 304: 917-933. doi: 10.1016/j.cej.2016.07.014
[15] LI X, CHEN D, LI N, et al. AgBr-loaded hollow porous carbon nitride with ultrahigh activity as visible light photocatalysts for water remediation[J]. Applied Catalysis B: Environmental, 2018, 229: 155-162. doi: 10.1016/j.apcatb.2018.02.028
[16] WANG S, GUAN B Y, LU Y, et al. Formation of hierarchical In2S3-CdIn2S4 heterostructured nanotubes for efficient and stable visible light CO2 reduction[J]. Journal of the American Chemical Society, 2017, 139(48): 17305-17308. doi: 10.1021/jacs.7b10733
[17] XU F, ZHU B, CHENG B, et al. 1D/2D TiO2/MoS2 hybrid nanostructures for enhanced photocatalytic CO2 reduction[J]. Advanced Optical Materials, 2018, 6(23): 1800911. doi: 10.1002/adom.201800911
[18] LOW J, DAI B, TONG T, et al. In situ irradiated X-ray photoelectron spectroscopy investigation on a direct Z-scheme TiO2/CdS composite film photocatalyst[J]. Advanced Materials, 2019, 31(6): 1802981. doi: 10.1002/adma.201802981
[19] MIYAUCHI M, IRIE H, LIU M, et al. Visible-light-sensitive photocatalysts: nanocluster-grafted titanium dioxide for indoor environmental remediation[J]. Journal of Physical Chemistry Letters, 2016, 7(1): 75-84. doi: 10.1021/acs.jpclett.5b02041
[20] 刘山虎, 许庆峰, 邢瑞敏, 等. 二氧化钛光催化技术应用于室内甲醛降解的研究进展[J]. 化学研究, 2016, 27(4): 502-513. doi: 10.14002/j.hxya.2016.04.020 [21] ZHU X, CHANG D L, LI X S, et al. Inherent rate constants and humidity impact factors of anatase TiO2 film in photocatalytic removal of formaldehyde from air[J]. Chemical Engineering Journal, 2015, 279: 897-903. doi: 10.1016/j.cej.2015.05.095
[22] MAMAGHANI A H, HAGHIGHAT F, LEE C S. Photocatalytic oxidation technology for indoor environment air purification: the state-of-the-art[J]. Applied Catalysis B: Environmental, 2017, 203: 247-269. doi: 10.1016/j.apcatb.2016.10.037
[23] PETER I J, VIGNESH G, VIJAYA S, et al. Enhancing the power conversion efficiency of SrTiO3/CdS/Bi2S3 quantum dot based solar cell using phosphor[J]. Applied Surface Science, 2019, 494: 551-560. doi: 10.1016/j.apsusc.2019.07.092
[24] HU B, CAI F, CHEN T, et al. Hydrothermal synthesis g-C3N4/Nano-InVO4 nanocomposites and enhanced photocatalytic activity for hydrogen production under visible light irradiation[J]. ACS Applied Materials & Interfaces, 2015, 7(33): 18247-18256.
[25] LIU S, XIA J, YU J. Amine-functionalized titanate nanosheet-assembled yolk@ shell microspheres for efficient cocatalyst-free visible-light photocatalytic CO2 reduction[J]. ACS Applied Materials & Interfaces, 2015, 7(15): 8166-8175.
[26] LIU X, YE M, ZHANG S, et al. Enhanced photocatalytic CO2 valorization over TiO2 hollow microspheres by synergetic surface tailoring and Au decoration[J]. Journal of Materials Chemistry A, 2018, 6(47): 24245-24255. doi: 10.1039/C8TA09661A
[27] WEON S, CHOI J, PARK T, et al. Freestanding doubly open-ended TiO2 nanotubes for efficient photocatalytic degradation of volatile organic compounds[J]. Applied Catalysis B: Environmental, 2017, 205: 386-392. doi: 10.1016/j.apcatb.2016.12.048
[28] FRIEDMANN D, HAKKI A, KIM H, et al. Heterogeneous photocatalytic organic synthesis: state-of-the-art and future perspectives[J]. Green Chemistry, 2016, 18(20): 5391-5411. doi: 10.1039/C6GC01582D
[29] SUN H, DONG B, SU G, et al. Modification of TiO2 nanotubes by WO3 species for improving their photocatalytic activity[J]. Applied Surface Science, 2015, 343: 181-187. doi: 10.1016/j.apsusc.2015.02.148
[30] BAJOROWICZ B, KOWALSKA E, NADOLNA J, et al. Preparation of CdS and Bi2S3 quantum dots co-decorated perovskite-type KNbO3 ternary heterostructure with improved visible light photocatalytic activity and stability for phenol degradation[J]. Dalton Transactions, 2018, 47(42): 15232-15245. doi: 10.1039/C8DT03094D
[31] LIU W, ZHONG D, DAI Z, et al. Synergetic utilization of photoabsorption and surface facet in crystalline/amorphous contacted BiOCl-Bi2S3 composite for photocatalytic degradation[J]. Journal of Alloys and Compounds, 2019, 780: 907-916. doi: 10.1016/j.jallcom.2018.12.003
[32] SHI H, WANG C, ZHAO Y, et al. Highly efficient visible light driven photocatalytic inactivation of E. coli with Ag QDs decorated Z-scheme Bi2S3/SnIn4S8 composite[J]. Applied Catalysis B: Environmental, 2019, 254: 403-413. doi: 10.1016/j.apcatb.2019.05.020
[33] NAUSHAD M, SHARMA G, ALOTHMAN Z A. Photodegradation of toxic dye using Gum Arabic-crosslinked-poly(acrylamide)/Ni(OH)2/FeOOH nanocomposites hydrogel[J]. Journal of Cleaner Production, 2019, 241: 118263. doi: 10.1016/j.jclepro.2019.118263
[34] LI F, LAN X, WANG L, et al. An efficient photocatalyst coating strategy for intimately coupled photocatalysis and biodegradation (ICPB): Powder spraying method[J]. Chemical Engineering Journal, 2020, 383: 123092. doi: 10.1016/j.cej.2019.123092
[35] 丁志伟, 张鹏, 刘玉民. CdS QDs/Bi2MoO6异质结光催化剂的制备及光催化性能研究[J]. 河南师范大学学报(自然科学版), 2020, 48(4): 58-65. https://www.cnki.com.cn/Article/CJFDTOTAL-HNSX202004010.htm [36] HOU Y, LI X, ZHAO Q, et al. Role of hydroxyl radicals and mechanism of Escherichia coli inactivation on Ag/AgBr/TiO2 nanotube array electrode under visible light irradiation[J]. Environmental Science & Technology, 2012, 46(7): 4042-4050.
[37] BAI S, ZHANG N, GAO C, et al. Defect engineering in photocatalytic materials[J]. Nano Energy, 2018, 53: 296-336. doi: 10.1016/j.nanoen.2018.08.058
[38] QU Y, DUAN X. Progress, challenge and perspective of heterogeneous photocatalysts[J]. Chemical Society Reviews, 2013, 42(7): 2568-2580. doi: 10.1039/C2CS35355E
[39] TERANISHI T, SAKAMOTO M. Charge separation in type-Ⅱ semiconductor heterodimers[J]. The Journal of Physical Chemistry Letters, 2013, 4(17): 2867-2873. doi: 10.1021/jz4013504
[40] 魏龙福, 余长林, 陈建钗, 等. 水热法合成Ag2CO3/ZnO异质结复合光催化剂及其光催化性能[J]. 有色金属科学与工程, 2014, 5(1): 47-47. doi: 10.13264/j.cnki.ysjskx.2014.01.009 [41] 薛霜霜, 何洪波, 吴榛, 等. 研磨-焙烧法制备BiOI/BiOBr异质结光催化剂及其光催化性能[J]. 有色金属科学与工程, 2017, 8(1): 86-88. doi: 10.13264/j.cnki.ysjskx.2017.01.015 [42] LOW J, JIANG C, CHENG B, et al. A review of direct Z-scheme photocatalysts[J]. Small Methods, 2017, 1(5): 1700080. doi: 10.1002/smtd.201700080
[43] KUMAR A, PRAJAPATI P K, PAL U, et al. Ternary rGO/InVO4/Fe2O3 Z-scheme heterostructured photocatalyst for CO2 reduction under visible light irradiation[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(7): 8201-8211.
[44] BHOSALE R, JAIN S, VINOD C P, et al. Direct Z-scheme g-C3N4/FeWO4 nanocomposite for enhanced and selective photocatalytic CO2 reduction under visible light[J]. ACS Applied Materials & Interfaces, 2019, 11(6): 6174-6183.
[45] HE F, MENG A, CHENG B, et al. Enhanced photocatalytic H2-production activity of WO3/TiO2 step-scheme heterojunction by graphene modification[J]. Chinese Journal of Catalysis, 2020, 41(1): 9-20. doi: 10.1016/S1872-2067(19)63382-6
[46] 黄海猛, 王常旺, 肖林昊. 非简并半导体中费米能级的简单计算及应用[J]. 大学物理, 2020, 39(1): 29-32. doi: 10.16854/j.cnki.1000-0712.190177 [47] XU C, LIU X, LI D, et al. Coordination of π-Delocalization in g-C3N4 for Efficient Photocatalytic Hydrogen Evolution under Visible Light[J]. ACS Applied Materials & Interfaces, 2021, 13(17): 20114-20124.
[48] LOW J, YU J, JARONIEC M, et al. Heterojunction photocatalysts[J]. Advanced Materials, 2017, 29(20): 1601694. doi: 10.1002/adma.201601694
[49] SUN S. Recent advances in hybrid Cu2O-based heterogeneous nanostructures[J]. Nanoscale, 2015, 7(25): 10850-10882. doi: 10.1039/C5NR02178B
[50] XIAO J, XIE Y, CAO H. Organic pollutants removal in wastewater by heterogeneous photocatalytic ozonation[J]. Chemosphere, 2015, 121: 1-17. doi: 10.1016/j.chemosphere.2014.10.072
[51] 曾德彬, 杨凯, 李笑笑, 等. Ag2CO3@AgBr复合光催化剂的制备、表征及其可见光催化性能[J]. 有色金属科学与工程, 2018, 9(1): 51-59. doi: 10.13264/j.cnki.ysjskx.2018.01.009 [52] LIU J J, CHENG B, YU J. A new understanding of the photocatalytic mechanism of the direct Z-scheme g-C3N4/TiO2 heterostructure[J]. Physical Chemistry Chemical Physics, 2016, 18(45): 31175-31183. doi: 10.1039/C6CP06147H
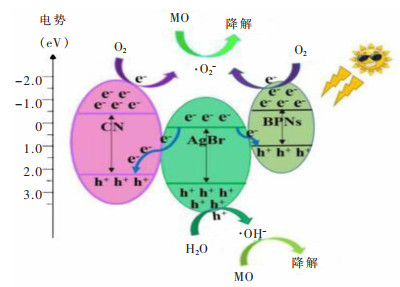
[53] ZENG D, YANG K, YU C, et al. Phase transformation and microwave hydrothermal guided a novel double Z-scheme ternary vanadate heterojunction with highly efficient photocatalytic performance[J]. Applied Catalysis B: Environmental, 2018, 237: 449-463. doi: 10.1016/j.apcatb.2018.06.010
[54] ZHOU D, CHEN Z, YANG Q, et al. Facile Construction of g-C3N4 Nanosheets/TiO2 Nanotube Arrays as Z-Scheme Photocatalyst with Enhanced Visible-Light Performance[J]. ChemCatChem, 2016, 8(19): 3064-3073. doi: 10.1002/cctc.201600828
[55] SHAO B, LIU X, LIU Z, et al. A novel double Z-scheme photocatalyst Ag3PO4/Bi2S3/Bi2O3 with enhanced visible-light photocatalytic performance for antibiotic degradation[J]. Chemical Engineering Journal, 2019, 368: 730-745. doi: 10.1016/j.cej.2019.03.013
[56] XU Q, ZHANG L, CHENG B, et al. S-Scheme Heterojunction Photocatalyst[J]. Chem, 2020, 6 (7): 1543-1559. doi: 10.1016/j.chempr.2020.06.010
[57] 樊谨菘, 陈静, 李江, 等. X射线辐照合成金纳米颗粒及其原位表征[J]. 辐射研究与辐射工艺学报, 2021, 39(4): 3-12. https://www.cnki.com.cn/Article/CJFDTOTAL-FYFG202104001.htm [58] BARR T L. Modern ESCA: The principles and practice of X-ray photoelectron spectroscopy[M]. New York: CRC Press, 2020.
[59] 王冰花, 陈金龙, 张彬. 原子力显微镜在高分子表征中的应用[J/OL]. 高分子学报: 1-15[2021-09-16]. [60] 张薇, 侯矍, 李楠, 等. 基于原子力显微镜的单分子力谱技术在高分子表征中的应用[J/OL]. 高分子学报: 1-24[2021-09-16]. [61] NOSAKA Y, NOSAKA A Y. Generation and detection of reactive oxygen species in photocatalysis[J]. Chemical Reviews, 2017, 117(17): 11302-11336. doi: 10.1021/acs.chemrev.7b00161
[62] DENG Y, ZHAO R. Advanced oxidation processes (AOPs) in wastewater treatment[J]. Current Pollution Reports, 2015, 1(3): 167-176. doi: 10.1007/s40726-015-0015-z
[63] HUANG C P, DONG C, TANG Z. Advanced chemical oxidation: its present role and potential future in hazardous waste treatment[J]. Waste Management, 1993, 13(5-7): 361-377. doi: 10.1016/0956-053X(93)90070-D
[64] LIANG Y H, LIAO M W, MISHRA M, et al. Fabrication of Ta3N5/ZnO direct Z-scheme photocatalyst for hydrogen generation[J]. International Journal of Hydrogen Energy, 2019, 44(35): 19162-19167. doi: 10.1016/j.ijhydene.2018.07.117
[65] WANG S, ZHU B, LIU M, et al. Direct Z-scheme ZnO/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity[J]. Applied Catalysis B: Environmental, 2019, 243: 19-26. doi: 10.1016/j.apcatb.2018.10.019
[66] XU F, ZHANG L, CHENG B, et al. Direct Z-scheme TiO2/NiS core-shell hybrid nanofibers with enhanced photocatalytic H2-production activity[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(9): 12291-12298.
[67] 王占国. 半导体材料研究的新进展[J]. 半导体技术, 2002, 8(3): 12-14. doi: 10.13290/j.cnki.bdtjs.2002.04.003 [68] 孙朝宁, 贺光辉, 赵振博, 等. 半导体材料国内外标准研究进展[J]. 中国标准化, 2021(15): 132-135, 146. doi: 10.3969/j.issn.1002-5944.2021.15.017 [69] SCHRODER D K. Semiconductor material and device characterization[M]. Phoenix Lieb press, 2009.
[70] SCHNEIDER J J, HOFFMANN R C, ENGSTLER J, et al. A printed and flexible field-effect transistor device with nanoscale zinc oxide as active semiconductor material[J]. Advanced Materials, 2008, 20(18): 3383-3387. doi: 10.1002/adma.200800819
[71] XU Q, ZHANG L, YU J, et al. Direct Z-scheme photocatalysts: principles, synthesis, and applications[J]. Materials Today, 2018, 21(10): 1042-1063. doi: 10.1016/j.mattod.2018.04.008
[72] ZHANG J, XU Q, FENG Z, et al. Importance of the relationship between surface phases and photocatalytic activity of TiO2[J]. Angewandte Chemie, 2008, 120(9): 1790-1793. doi: 10.1002/ange.200704788
[73] DONG H, ZHANG X, LI J, et al. Construction of morphology-controlled nonmetal 2D/3D homojunction towards enhancing photocatalytic activty and mechanism insight[J]. Applied Catalysis B: Environmental, 2020, 263: 118270. doi: 10.1016/j.apcatb.2019.118270
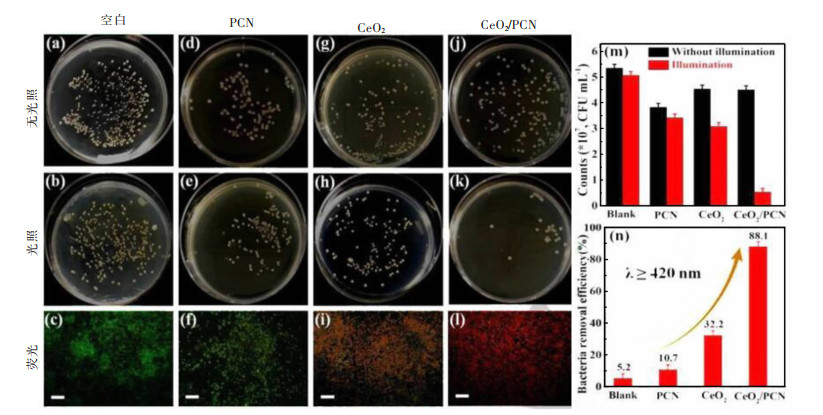
[74] 温福宇, 杨金辉, 宗旭, 等. 太阳能光催化制氢研究进展[J]. 化学进展, 2009, 21(11): 2285-2302. https://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ200911005.htm [75] WANG Q, HISATOMI T, JIA Q, et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%[J]. Nature Materials, 2016, 15(6): 611. doi: 10.1038/nmat4589
[76] KUMAR S S, HIMABINDU V. Hydrogen production by PEM water electrolysis-A review[J]. Materials Science for Energy Technologies, 2019, 2(3): 442-454. doi: 10.1016/j.mset.2019.03.002
[77] DAWOOD F, ANDA M, SHAFIULLAH G M. Hydrogen production for energy: An overview[J]. International Journal of Hydrogen Energy, 2020, 45(7): 3847-3869. doi: 10.1016/j.ijhydene.2019.12.059
[78] ZHANG S, FAN Q, XIA R, et al. CO2 reduction: from homogeneous to heterogeneous electrocatalysis[J]. Accounts of Chemical Research, 2020, 53(1): 255-264. doi: 10.1021/acs.accounts.9b00496
[79] RANRAN J, JARONIEC M, QIAO S Z. Cocatalysts in semiconductor-based photocatalytic CO2 reduction: achievements, challenges, and opportunities[J]. Advanced Materials, 2018, 30(7): 1704649. doi: 10.1002/adma.201704649
[80] KHAKI M R D, SHAFEEYAN M S, RAMAN A A A, et al. Application of doped photocatalysts for organic pollutant degradation-A review[J]. Journal of Environmental Management, 2017, 198: 78-94.
[81] HUANG B C, JIANG J, HUANG G X, et al. Sludge biochar-based catalysts for improved pollutant degradation by activating peroxymonosulfate[J]. Journal of Materials Chemistry A, 2018, 6(19): 8978-8985. doi: 10.1039/C8TA02282H
[82] LIU H, MA S, SHAO L, et al. Defective engineering in graphitic carbon nitride nanosheet for efficient photocatalytic pathogenic bacteria disinfection[J]. Applied Catalysis B: Environmental, 2020, 261: 118201. doi: 10.1016/j.apcatb.2019.118201
[83] YU N, WANG X, QIU L, et al. Bacteria-triggered hyaluronan/AgNPs/gentamicin nanocarrier for synergistic bacteria disinfection and wound healing application[J]. Chemical Engineering Journal, 2020, 380: 122582. doi: 10.1016/j.cej.2019.122582
[84] LI X, KANG B, DONG F, et al. Enhanced photocatalytic degradation and H2/H2O2 production performance of S-pCN/WO2.72 S-scheme heterojunction with appropriate surface oxygen vacancies[J]. Nano Energy, 2021, 81: 105671-105679. doi: 10.1016/j.nanoen.2020.105671
[85] FU J, XU Q, LOW J, et al. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst[J]. Applied Catalysis B: Environmental, 2019, 243: 556-565. doi: 10.1016/j.apcatb.2018.11.011
[86] CHEN Y, SU F, XIE H, et al. One-step construction of S-scheme heterojunctions of N-doped MoS2 and S-doped g-C3N4 for enhanced photocatalytic hydrogen evolution[J]. Chemical Engineering Journal, 2021, 404: 126498. doi: 10.1016/j.cej.2020.126498
[87] ZHEN Y, YANG C, SHEN H, et al. Photocatalytic performance and mechanism insights of a S-scheme g-C3N4/Bi2MoO6 heterostructure in phenol degradation and hydrogen evolution reactions under visible light[J]. Phys Chem Chem Phys, 2020, 22 (45): 26278-26288. doi: 10.1039/D0CP02199G
[88] LI H, WANG G, GONG H, et al. Hollow nanorods and amorphous Co9S8 quantum dots construct S-scheme heterojunction for efficient hydrogen evolution[J]. The Journal of Physical Chemistry C, 2021, 125(1): 648-659. doi: 10.1021/acs.jpcc.0c10239
[89] GE H, XU F, CHENG B, et al. S-scheme heterojunction TiO2/CdS nanocomposite nanofiber as H2-production photocatalyst[J]. Chem Cat Chem, 2019, 11(24): 6301-6309.
[90] HU T, DAI K, ZHANG J, et al. Noble-metal-free Ni2P modified step-scheme SnNb2O6/CdS-diethylenetriamine for photocatalytic hydrogen production under broadband light irradiation[J]. Applied Catalysis B: Environmental, 2020, 269: 118844. doi: 10.1016/j.apcatb.2020.118844
[91] XU Q, MA D, YANG S, et al. Novel g-C3N4/g-C3N4 S-scheme isotype heterojunction for improved photocatalytic hydrogen generation[J]. Applied Surface Science, 2019, 495: 143555. doi: 10.1016/j.apsusc.2019.143555
[92] 梅子慧, 王国宏, 严素定, 等. 微波辅助快速制备2D/1D ZnIn2S4/TiO2 S-型异质结及其光催化制氢性能[J]. 物理化学学报, 2020, 37(6): 2009097-0. https://www.cnki.com.cn/Article/CJFDTOTAL-WLHX202106011.htm [93] 张梦凡, 张振民, 贾静雯, 等. Z-型异质结光催化剂的设计、制备和应用研究进展[J]. 有色金属科学与工程, 2020, 11(3): 18-32. doi: 10.13264/j.cnki.ysjskx.2020.03.003 [94] HUO Y, ZHANG J, DAI K, et al. Amine-modified S-scheme porous g-C3N4/CdSe-diethylenetriamine composite with enhanced photocatalytic CO2 reduction activity[J]. ACS Applied Energy Materials, 2021, 4(1): 956-968.
[95] XIE Q, HE W, LIU S, et al. Bifunctional S-scheme g-C3N4/Bi/BiVO4 hybrid photocatalysts toward artificial carbon cycling[J]. Chinese Journal of Catalysis, 2020, 41(1): 140-153.
[96] WANG Z, CHEN Y, ZHANG L, et al. Step-scheme CdS/TiO2 nanocomposite hollow microsphere with enhanced photocatalytic CO2 reduction activity[J]. Journal of Materials Science Technology, 2020, 56: 143-150.
[97] DENG H, FEI X, YANG Y, et al. S-scheme heterojunction based on p-type ZnMn2O4 and n-type ZnO with improved photocatalytic CO2 reduction activity[J]. Chemical Engineering Journal, 2021, 409.
[98] HE F, ZHU B, CHENG B, et al. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO2 reduction activity[J]. Applied Catalysis B: Environmental, 2020, 272: 119006.
[99] HUO Y, ZHANG J, WANG Z, et al. Efficient interfacial charge transfer of 2D/2D porous carbon nitride/bismuth oxychloride step-scheme heterojunction for boosted solar-driven CO2 reduction[J]. Journal of Colloid and Interface Science, 2021, 585: 684-693.
[100] WANG P, LIU Y, JIANG N, et al. Double S-scheme AgBr heterojunction co-modified with g-C3N4 and black phosphorus nanosheets greatly improves the photocatalytic activity and stability[J]. Journal of Molecular Liquids, 2021, 329: 115540.
[101] WU S, YU X, ZHANG J, et al. Construction of BiOCl/CuBi2O4 S-scheme heterojunction with oxygen vacancy for enhanced photocatalytic diclofenac degradation and nitric oxide removal[J]. Chemical Engineering Journal, 2021, 411: 128555.
[102] JIA X, HAN Q, LIU H, et al. A dual strategy to construct flowerlike S-scheme BiOBr/BiOAc1-Br heterojunction with enhanced visible-light photocatalytic activity[J]. Chemical Engineering Journal, 2020, 399: 125701.
[103] CHEN J, LIU T, ZHANG H, et al. One-pot preparation of double S-scheme Bi2S3/MoO3/C3N4 heterojunctions with enhanced photocatalytic activity originated from the effective charge pairs partition and migration[J]. Applied Surface Science, 2020, 527: 146788.
[104] WU Y, SONG M, CHAI Z, et al. Integrating an Ag0-Ag+ mediated Ag2Ta4O11/Ag8(Nb0.5Ta0.5)26O69 heterojunction to quickly decontaminate indoor gaseous formaldehyde under indoor temperature, humidity and sunlight irradiation conditions[J]. Environmental Science: Nano, 2020, 7(6): 1831-1840.
[105] MENG S, SUN W, ZHANG S, et al. Insight into the Transfer Mechanism of Photogenerated Carriers for WO3/TiO2 Heterojunction Photocatalysts: Is It the Transfer of Band-Band or Z-Scheme Why[J]. The Journal of Physical Chemistry C, 2018, 122 (46): 26326-26336.
[106] RONGAN H, HAIJUAN L, HUIMIN L, et al. S-scheme photocatalyst Bi2O3/TiO2 nanofiber with improved photocatalytic performance[J]. Journal of Materials Science & Technology, 2020, 52: 145-151.
[107] ZHANG K, ZHOU M, YU C, et al. Construction of S-scheme g-C3N4/ZrO2 heterostructures for enhancing photocatalytic disposals of pollutants and electrocatalytic hydrogen evolution[J]. Dyes and Pigments, 2020, 180: 108525.
[108] ZHANG B, SHI H, HU X, et al. A novel S-scheme MoS2/CdIn2S4 flower-like heterojunctions with enhanced photocatalytic degradation and H2 evolution activity[J]. Journal of Physics D: Applied Physics, 2020, 53 (20): 205101.
[109] UNUABONAH E I, UGWUJA C G, OMOROGIE M O, et al. Clays for efficient disinfection of bacteria in water[J]. Applied Clay Science, 2018, 151: 211-223.
[110] DING W, JIN W, CAO S, et al. Ozone disinfection of chlorine-resistant bacteria in drinking water[J]. Water Research, 2019, 160: 339-349.
[111] LIU H, MA S, SHAO L, et al. Defective engineering in graphitic carbon nitride nanosheet for efficient photocatalytic pathogenic bacteria disinfection[J]. Applied Catalysis B: Environmental, 2020, 261: 118201.
[112] LIN K, MARR L C. Humidity-dependent decay of viruses, but not bacteria, in aerosols and droplets follows disinfection kinetics[J]. Environmental Science & Technology, 2019, 54(2): 1024-1032.
[113] DENG J, LIANG J, LI M, et al. Enhanced visible-light-driven photocatalytic bacteria disinfection by g-C3N4-AgBr[J]. Colloids and Surfaces B: Biointerfaces, 2017, 152: 49-57.
[114] HOU C, HE W, WANG Z, et al. Particulate-aggregated adhesives with exudate-sensitive properties and sustained bacteria disinfection to facilitate wound healing[J]. ACS Applied Materials & Interfaces, 2020, 12(28): 31090-31098.
[115] WANG W, HUANG G, JIMMY C Y, et al. Advances in photocatalytic disinfection of bacteria: development of photocatalysts and mechanisms[J]. Journal of Environmental Sciences, 2015, 34: 232-247.
[116] XIA P, CAO S, ZHU B, et al. Designing a 0D/2D S-scheme heterojunction over polymeric carbon nitride for visible-light photocatalytic inactivation of bacteria[J]. Angewandte Chemie International Edition, 2020, 59(13): 5218-5225.



 下载:
下载: