Research progress in the design, fabrication and application of Z-scheme heterojunction photocatalysts
-
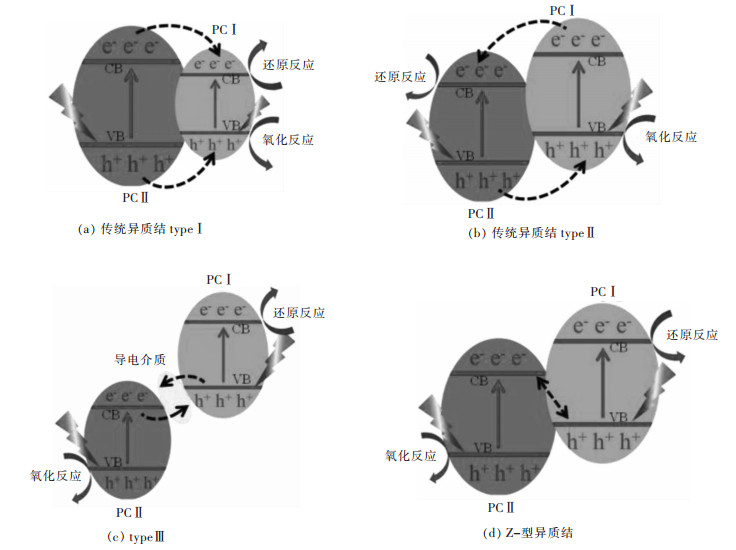
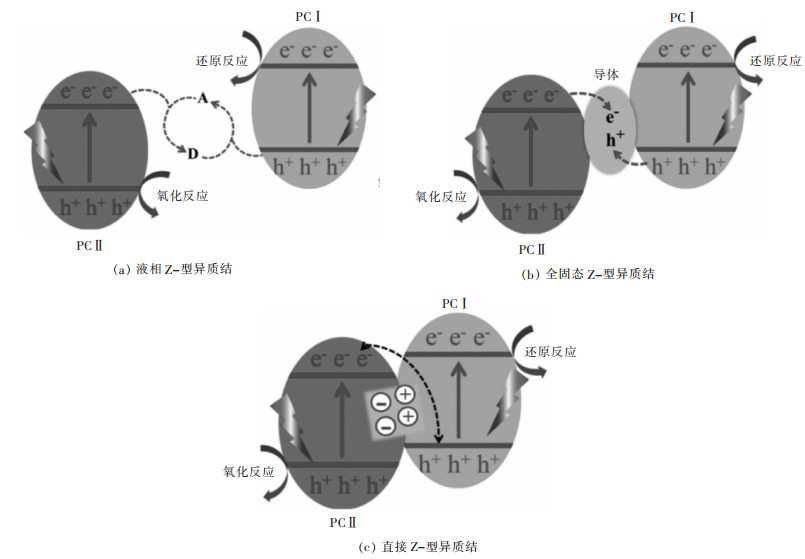
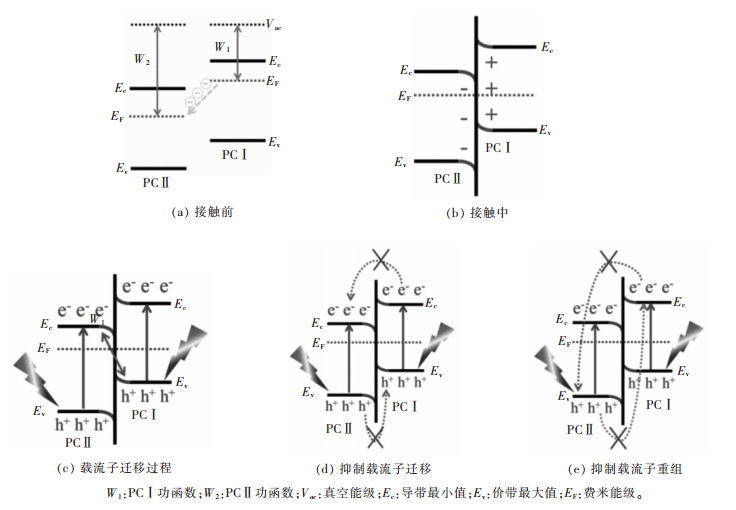
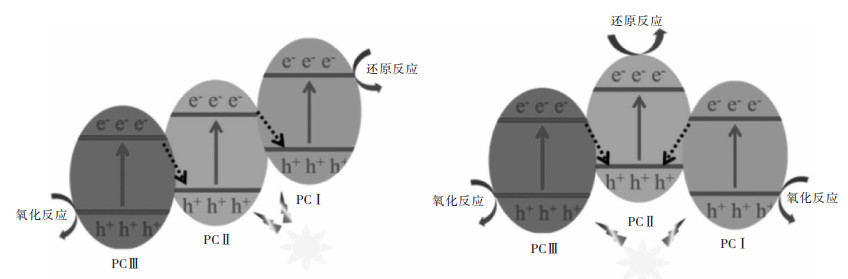
摘要: 传统异质结具有扩大光响应范围、促进载流子分离的优点,但存在氧化-还原能力不够的问题。Z-型异质结是根据自然界植物光合作用模拟的人工光合作用而提出的,相对于单一光催化剂与传统异质结光催化剂具有能有效分离电子空穴对、减少复合几率、保留强氧化-还原活性位点、扩大光响应范围、提高光催化活性等优点。文中综述了近年来,液相Z-型异质结光催化剂、全固态Z-型异质结光催化剂、直接Z-型异质结光催化剂的反应机理、构建方法与在光解水产氢、CO2还原、有机物的降解、水中重金属离子的还原等应用方面的研究进展。并对比几类Z-型异质结光催化剂的特点,提出了Z-型光催化体系发展的未来挑战和前景。Abstract: Traditional heterojunction boasts such advantages as enlargement in optical response range and facilitation in carrier separation. However, its redox ability is insufficient. Z-scheme heterojunction is put forward based on artificial photosynthesis, a simulation of plant photosynthesis in nature. With respect to the single photocatalyst and traditional heterojunction, Z-scheme heterojunction has distinct advantages, e.g. the effective separation of electron-hole pairs, reduced recombination of photo-induced carrier, strong redox active site, expanded light response range, high photocatalytic activity, etc. In this paper, the reaction mechanism, construction method and application of liquid phase Z-scheme heterojunction, all-solid-state Z-scheme heterojunction and direct Z-scheme heterojunction are reviewed. Finally, the characteristics of several types of Z-scheme heterojunction were briefly summarized and compared, and the challenges and prospects for the development of Z-scheme photocatalytic systems are proposed.
-
铷是重要的战略碱金属,具有优异的物理、化学和光电性能[1-3]。长期以来,铷及其化合物在原子钟、光电池、特种玻璃、生物化学及医药等传统领域中有着重要用途[4]。近年来,在钙钛矿电池、动力发电、离子推进发动机、激光能转换电能装置等新兴应用领域中[5-8],铷也表现出越来越重要的作用,显示出强劲的生命力。因此,铷的提取和应用已引起世界各国的广泛关注。
自然界中的铷资源主要分布在盐湖卤水和矿石中,在地壳中的丰度排在第16位[9-12]。铷比同主族的碱金属锂和铯以及许多常见的金属锌、铅等的储量更丰富,但是这些金属每年的开采量却数倍于铷[13-14]。这是因为铷的分布分散且常以微量存在,开采难度较大。并且铷鲜有自己独立存在的矿物,主要以类质同象的形式取代钾的位置存在于花岗岩、光卤石和花岗伟晶岩类矿物中[15]。一直以来,铷主要是从铯榴石和锂云母提取铯、锂的副产物中回收,资源回收率低。近年来,一些文献报道的矿石提铷工艺主要可以分为:焙烧分解法、酸分解法以及酸碱联合法[16]。其中,焙烧分解法以氯化焙烧法为主,是目前研究最多的方法。氯化焙烧法的原理是氯化物与矿物中的碱金属会在高温下发生反应,产生氯化氢气体。氯化氢气体继续与含铷云母反应,破坏云母稳定的结构,使其中的铷释放出来,最后通过水浸使可溶性的氯化铷进入溶液[17-18]。氯化焙烧法虽然可以将铷高效浸出,但未考虑矿物中丰富硅、铝资源的资源化利用,并且采用氯化焙烧会产生大量的含盐酸废气和高盐废水,处理技术难度较大,成本较高。酸碱联合法[19]是目前得到广泛关注的矿石提铷方法,该法克服了酸法只能高效浸出云母而不能有效破坏长石结构的缺点。并且酸碱联合法不仅考虑到了铷的高效回收,还考虑到了宏量元素硅、铝的资源化利用,浸出液返回浸出,避免了大量高盐废水的产生。但是酸碱联合法流程较长,浸出压力和碱耗较高,仍然具有优化的可能[19-20]。
为了充分破坏含铷矿物中稳定的硅氧四面体结构,有效降低浸出压力和碱耗。课题组提出了对铷矿进行熔融水淬处理,在高温下强制破坏矿物结构,使铷彻底解离,实现其高效浸出[21-22]。为了进一步研究所得水淬渣的浸出活性,系统研究碱浸过程中氢氧化钠浓度、温度和水淬渣粒度对浸出速率的影响,分析水淬渣浸出动力学和浸出控制性环节,以期为复杂铷矿的高效处理提供参考。
1 试验
1.1 试验原料
铷矿熔融水淬渣是由铷矿与质量分数30%的氢氧化钠充分混合后,在1 250 ℃条件下焙烧2 h直接水淬所得。铷矿与熔融水淬渣的主要成分如表 1所列。氢氧化钠质量分数为96%,为分析纯。
表 1 钨酸铵原料液中各杂质元素的浓度Table 1. The concentration of each impurity element in ammonium tungstate feed liquid
1.2 试验仪器
试验所使用的仪器主要有SHJ-A6恒温磁力搅拌水浴锅、PL203电子天平、SHB-3循环水式真空泵、DZF-6090真空干燥箱、烧杯、容量瓶、量筒、玻璃杯、移液管等。
1.3 试验步骤与分析手段
首先配置500 mL一定浓度的氢氧化钠溶液于烧杯中,将烧杯放入恒温水浴锅中加热至设定温度后加入10 g细磨水淬渣,调节转速为400 r/min开始反应。每隔2 min取5 mL浆液,液固分离后,对滤液中的铷含量进行分析。实验中,水淬渣、浸出液和浸出渣中的铷离子浓度通过电感耦合等离子发射光谱(ICP)进行分析,水淬渣和浸出渣的物相、形貌通过X射线衍射仪(XRD)、扫描电镜(SEM)、能谱仪(EDS)等方法进行分析,水淬渣粒度通过激光粒度分析仪进行分析。
2 结果与讨论
2.1 铷矿熔融水淬渣分析
熔融一段时间后,铷矿中稳定的硅酸盐结构被强制破坏,直接水淬使矿物保持高温下高活性状态,得到的水淬渣以高活性的状态存在。水淬渣的XRD图谱如图 1所示,从图 1可以看出,水淬渣无晶型,以无定形的状态存在,与预期相符。
为了进一步分析经过熔融焙烧后各有价元素的赋存状态,对熔融水淬渣进行了SEM-EDS分析,所得结果如图 2所示。从水淬渣的能谱图中可以看出,铷以高度分散的状态存在,可以进一步推测在前期熔融阶段原矿的稳定物相已经被充分破坏。
2.2 碱浸实验
2.2.1 氢氧化钠浓度对铷浸出率的影响
取10 g细磨至38~48 μm之间的水淬渣,在碱浸温度100 ℃,液固比50:1 (mL/g),转速400 r/min的条件下,研究氢氧化钠浓度(100,160,180,200 g/L)对铷浸出的影响,结果如图 3所示。从图 3中可以看出氢氧化钠浓度对水淬渣的浸出影响较为明显。随着氢氧化钠浓度的增加,铷的浸出率快速上升。在氢氧化钠浓度为200 g/L条件下,反应10 min,铷浸出率达到65%以上。熔融水淬-碱浸法相较于现阶段碱法对铷矿的处理工艺中碱耗较高(氢氧化钠浓度为600 g/L)的问题,具有明显的优势[22]。
2.2.2 碱浸温度对铷浸出率的影响
取10 g细磨至38~48 μm之间的水淬渣,在氢氧化钠浓度为200 g/L,液固比50:1 (mL/g), 转速400 r/min的条件下,研究碱浸温度(45,70,90,100 ℃)对铷浸出的影响。不同碱浸温度下,铷的浸出率与时间的关系如图 4所示。从图 4中可以看出浸出温度对水淬渣的浸出影响较为显著。温度可以有效的影响分子的热运动,温度越高,分子的热运动越快,单位时间参与浸出反应的分子越多,浸出速度越快。碱浸反应在100 ℃下反应速率很快,反应10 min,铷浸出率就能达到65%左右。该法相比较现阶段碱法工艺中存在的碱浸温度较高(约250 ℃)的问题,也具有较大优势[22]。
2.2.3 水淬渣粒度对铷浸出率的影响
取10 g水淬渣,在碱浸温度为100 ℃,液固比为50:1 (mL/g),转速400 r/min,氢氧化钠浓度为200 g/L的条件下,研究水淬渣粒度(25~38,38~48,48~75,75~150 μm)对铷浸出效果的影响,结果如图 5所示。
从图 5中可以看出,水淬渣粒度对铷浸出率影响较为明显,在一定范围内,水淬渣粒度越小,浸出速率更快,铷浸出率越高。但是当粒度小于38~48 μm时,继续减小,水淬渣的浸出速率变化不大。因此可以适当减小水淬渣粒度来获得更好的浸出效果。
2.3 浸出动力学分析
铷矿水淬渣的浸出本质上为非均相液固反应,假设水淬渣的碱浸反应动力学过程可以用未反应核模型来描述[23-25]。水淬渣的碱浸主要包括以下①~⑤共5个过程:①浸出剂从溶液中运动到固体产物层表面(外扩散);②浸出剂离子通过固体产物层向反应界面的扩散(内扩散);③浸出剂离子在反应界面与未反应水淬渣发生化学反应(化学反应);④反应产物通过从反应界面通过固体产物层向边界层的扩散(内扩散);⑤碱浸渣从固体颗粒表面扩散到浸出液中(外扩散)。
上述不同控制步骤的控速方程如下:

(1) 
(2) 
(3) 
(4) 其中k1,k2,k3,k4是不同控速环节的速率常数,分别对应外扩散控制、化学反应控制、内扩散控制和混合控制的速率常数;x是铷的浸出率,%;t为碱浸反应的时间,min。
2.3.1 温度对浸出过程影响的动力学分析
将不同温度下铷的浸出率分别带入上述未反应核模型的不同动力学控制方程,并进行线性拟合,拟合效果如图 6所示,各模型对应温度下的拟合优度如表 2所列。分析表 2拟合结果,外扩散控制模型、化学反应控制模型的拟合优度均小于0.90,内扩散控制和混合控制的模型在拟合优度均在0.95以上。为了进一步确定该浸出反应的控速方程,通过Arrhenius[23, 26]方程如式(5)对浸出过程进一步分析。
表 2 杂质S初始浓度对其析出率及在APT中的含量的影响Table 2. Effect of initial concentration of impurity S on itself precipitation rate and content in APT crystal
通过图 6中各浸出温度下直线的斜率确定速率常数k (如表 2),并带入Arrhenius方程计算浸出反应的表观活化能。

(5) 式(5)中:k为速常数;A为指前因子;Ea为浸出反应的表观活化能,kJ/mol;R为摩尔气体常数,J/(mol·K);T为热力学温度,K。对Arrhenius方程两边取对数,构建lnk与1 000/T的关系(图 7)。通过图 7中Arrhenius曲线可以求出内扩散控制下浸出反应的表观活化能为Ea=33.25 kJ/mol,而扩散控制模型的表观活化能小于20 kJ/mol。因此,铷的浸出不属于内扩散控制[23]。混合控制下计算出的浸出反应的表观活化能为Ea=37.41 kJ/mol,表观活化能属于混合控制下的活化能的数值区间(20~40 kJ/mol),这也进一步证明了铷矿水淬渣的浸出过程符合混合控制的动力学模型。
2.3.2 水淬渣粒度对浸出过程影响的动力学分析
把不同粒度水淬渣对应铷的浸出率带入混合控制的动力学方程并进行线性拟合(如图 8)。分析不同水淬渣粒度条件下对混合控制模型的拟合优度(如表 3),可以看出混合控制模型的拟合优度均在0.95以上,与上文中得出的符合混合控制模型的结论一致。
表 3 不同水淬渣粒度条件下混合控制模型的拟合数据Table 3. Fitting data of the hybrid control model for different water quenched slag particle sizes
经查阅文献[23, 27],符合收缩核模型的动力学过程,还满足克兰克-金斯特林-布劳希特因方程式(6)。其中D为浸出剂的有效扩散系数;M为氢氧化钠的相对分子质量;C为浸出液中氢氧化钠浓度;δ为化学反应式中反应物的计量系数;ρ为反应物密度;r0为水淬渣原始半径,且当只改变水淬渣粒径时D,M,δ,C,ρ均视为常数。对式(6)两边取自然对数,并作lnk与lnd0(d0为水淬渣粒径)的关系图,如图 9所示。

(6) 从图 9中可以看出,lnk与lnd0成很好的线性关系,拟合优度为0.98,水淬渣的粒径对碱浸反应的反应级数为-0.86,碱浸过程满足克兰克-金斯特林-布劳希特因方程,这也证明铷矿水淬渣的碱浸过程符合混合控制模型,并且在一定范围内降低铷矿水淬渣粒径可以有效提高铷的浸出速率。
2.4 动力学方程的确立
从上述实验中可以看出,碱浸过程中的浸出温度和水淬渣粒度均会对浸出过程产生较大影响。因此,浸出过程在浸出温度和水淬渣粒度作用下未反应核模型的表观反应速率常数可以表示为式(7)[28]。

(7) 式(7)中:A为Arrhenius方程的指前因子a为水淬渣粒度的反应级数。从图 7和图 9中的拟合结果可以得出表观活化能为37.41 kJ/mol,A=321和a=-0.86,代入式(4)可得混合控制模型的反应动力学方程为:

(8) 2.5 碱浸过程物相分析
为了分析碱浸过程水淬渣的物相转变及形貌变化,对碱浸渣(氢氧化钠200 g/L,98 ℃,1 h)进行了XRD以及扫描电镜分析。通过对图 10和图 11进行分析可以得出,经过碱浸,无定形的水淬渣转变为晶型较好的方钠石。生成的方钠石是由许多个表面疏松、比表面积较大的小球团簇在一起组成。方钠石作为类沸石类矿物,具有典型的硅酸盐矿物骨架。并且方钠石与A型沸石和X型沸石具有相同的β特征笼,可以在合适的条件下通过简单的沸石转化法进行转化[29],进而实现对矿物中硅、铝资源的高值化利用。
3 结论
通过对铷矿水淬渣系统的实验研究,得出了以下结论:
1) 结构稳定的铷矿经过熔融水淬后,稳定的结构被破坏,水淬渣的浸出活性较高。熔融水淬碱浸工艺相较于现阶段铷矿的碱法提铷工艺,具有碱耗和浸出温度较低的优势,可以实现在低碱、低温条件下铷的高效浸出。
2) 铷矿水淬渣的浸出动力学过程符合混合控制模型,拟合优度达到0.95以上,浸出过程的表观活化能为37.41 kJ/mol,混合控制模型的动力学方程为:ln(1-x)/3+(1-x)-1/3-1=321·d0-0.86·exp[-33250/(RT)]·t。
3) 碱浸过程中氢氧化钠浓度、浸出温度和水淬渣粒度是影响水淬渣浸出速率的最主要因素。在适当减小水淬渣粒径条件下,提高温度和氢氧化钠浓度可以有效提高铷的浸出率。
-
表 1 Z-型异质结构建方法
Table 1 Construction method of Z-scheme heterojunction

表 2 用于光解水产氢产氧的Z-型异质结光催化剂
Table 2 Hydrogen and oxygen production over Z-scheme heterojunction photocatalysts

表 3 CO2还原产物及电势
Table 3 CO2 reduction products and potential

表 4 用于CO2还原的Z-型异质结光催化剂
Table 4 Reduction of CO2 over Z-scheme heterojunction photocatalysts

表 5 用于降解有机污染物的Z-型异质结光催化剂
Table 5 Degradation of organic pollutants over Z-scheme heterojunction photocatalysts

-
[1] BAHRUDIN N N, NAWI M A. Immobilized titanium dioxide/powdered activated carbon system for the photocatalytic adsorptive removal of phenol[J]. Korean Journal of Chemical Engineering, 2018, 35(7): 1532-1541.2. doi: 10.1007/s11814-018-0062-4
[2] MURRAY J, KING D. Climate policy: Oil's tipping point has passed[J]. Nature, 2012, 481(7382): 433. doi: 10.1038/481433a
[3] KONG L, MU X, FAN0 X, et al. Site-selected N vacancy of g-C3N4 for photocatalysis and physical mechanism[J]. Applied Materials Today, 2018, 13: 329-338. doi: 10.1016/j.apmt.2018.10.003
[4] MENG F, LIU Y, WANG J, et al. Temperature dependent photocatalysis of g-C3N4, TiO2 and ZnO: Differences in photoactive mechanism[J]. Journal of Colloid and Interface Science, 2018, 532: 321-330. doi: 10.1016/j.jcis.2018.07.131
[5] FUJISHIMA A, HONDA K. Electrochemical photolysis of water at a semiconductor electrode[J]. Nature, 1972, 238(5358): 37-38. doi: 10.1038/238037a0
[6] YU C, HE H, FAN Q, et al. Novel B-doped BiOCl nanosheets with exposed (001) facets and photocatalytic mechanism of enhanced degradation efficiency for organic pollutants[J]. Science of the Total Environment, 2019, 694: 133727. doi: 10.1016/j.scitotenv.2019.133727
[7] YU C, HE H, LIU X, et al. Novel SiO2 nanoparticle-decorated BiOCl nanosheets exhibiting high photocatalytic performances for the removal of organic pollutants[J]. Chinese Journal of Catalysis, 2019, 40(8): 1212-1221. doi: 10.1016/S1872-2067(19)63359-0
[8] YU C, WU Z, LIU R, et al. Novel fluorinated Bi2MoO6 nanocrystals for efficient photocatalytic removal of water organic pollutants under different light source illumination[J]. Applied Catalysis B: Environmental, 2017, 209: 1-11. doi: 10.1016/j.apcatb.2017.02.057
[9] YANG K, LI X, YU C, et al. Review on heterophase/homophase junctions for efficient photocatalysis: The case of phase transition construction[J]. Chinese Journal of Catalysis, 2019, 40(6): 796-818. doi: 10.1016/S1872-2067(19)63290-0
[10] YU C, ZHOU W, ZHU L, et al. Integrating plasmonic Au nanorods with dendritic like α-Bi2O3/Bi2O2CO3 heterostructures for superior visible-light-driven photocatalysis[J]. Applied Catalysis B: Environmental, 2016, 184: 1-11. doi: 10.1016/j.apcatb.2015.11.026
[11] TIAN J, WU Z, LIU Z, et al. Low-cost and efficient visible-light-driven CaMg(CO3)2@Ag2CO3 microspheres fabricated via an ion exchange route[J]. Chinese Journal of Catalysis, 2017, 38(11): 1899-1908. doi: 10.1016/S1872-2067(17)62924-3
[12] PICHAT P. A brief survey of the practicality of using photocatalysis to purify the ambient air (indoors or outdoors) or air effluents[J]. Applied Catalysis B: Environmental, 2019, 245: 770-776. doi: 10.1016/j.apcatb.2018.12.027
[13] REN H, KOSHY P, CHEN W F, et al. Photocatalytic materials and technologies for air purification[J]. Journal of Hazardous Materials, 2017, 325: 340-366. doi: 10.1016/j.jhazmat.2016.08.072
[14] CHA B J, SAQLAIN S, SEO H O, et al. Hydrophilic surface modification of TiO2 to produce a highly sustainable photocatalyst for outdoor air purification[J]. Applied Surface Science, 2019, 479: 31-38. doi: 10.1016/j.apsusc.2019.01.261
[15] FANG Y, MA Y, ZHENG M, et al. Metal-organic frameworks for solar energy conversion by photoredox catalysis[J]. Coordination Chemistry Reviews, 2018, 373: 83-115. doi: 10.1016/j.ccr.2017.09.013
[16] JIN X, YE L, XIE H, et al. Bismuth-rich bismuth oxyhalides for environmental and energy photocatalysis[J]. Coordination Chemistry Reviews, 2017, 349: 84-101. doi: 10.1016/j.ccr.2017.08.010
[17] PHURUANGRAT A, SIRI S, WADBUA P, et al. Microwave-assisted synthesis, photocatalysis and antibacterial activity of Ag nanoparticles supported on ZnO flowers[J]. Journal of Physics and Chemistry of Solids, 2019, 126: 170-177. doi: 10.1016/j.jpcs.2018.11.007
[18] YIN H, CHEN X, LI G, et al. Sub-lethal photocatalysis bactericidal technology cause longer persistence of antibiotic-resistance mutant and plasmid through the mechanism of reduced fitness cost[J]. Applied Catalysis B: Environmental, 2019, 245: 698-705. doi: 10.1016/j.apcatb.2019.01.041
[19] LEE M, SHAHBAZ H M, KIM J U, et al. Efficacy of UV-TiO2 photocatalysis technology for inactivation of Escherichia coli K12 on the surface of blueberries and a model agar matrix and the influence of surface characteristics[J]. Food Microbiology, 2018, 76: 526-532. doi: 10.1016/j.fm.2018.07.015
[20] YAO C, YUAN A, ZHANG H, et al. Facile surface modification of textiles with photocatalytic carbon nitride nanosheets and the excellent performance for self-cleaning and degradation of gaseous formaldehyde[J]. Journal of Colloid and Interface Science, 2019, 533: 144-153. doi: 10.1016/j.jcis.2018.08.058
[21] BANERJEE S, DIONYSIOU D D, PILLAI S C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis[J]. Applied Catalysis B: Environmental, 2015, 176: 396-428. https://www.sciencedirect.com/science/article/pii/S0926337315001794
[22] FAN Y, ZHOU J, ZHANG J, et al. Photocatalysis and self-cleaning from g-C3N4 coated cotton fabrics under sunlight irradiation[J]. Chemical Physics Letters, 2018, 699: 146-154. doi: 10.1016/j.cplett.2018.03.048
[23] NAUFAL B, ULLATTIL S G, PERIYAT P. A dual function nanocrystalline TiO2 platform for solar photocatalysis and self cleaning application[J]. Solar Energy, 2017, 155: 1380-1388. doi: 10.1016/j.solener.2017.08.005
[24] ZHOU P, YU, JARONIEC M. All-solid-state Z-scheme photocatalytic systems[J]. Advanced Materials, 2014, 26(29): 4920-4935. doi: 10.1002/adma.201400288
[25] QI K, CHENG B, YU J, et al. A review on TiO2-based Z-scheme photocatalysts[J]. Chinese Journal of Catalysis, 2017, 38(12): 1936-1955. doi: 10.1016/S1872-2067(17)62962-0
[26] LOW J, YU J, JARONIEC M, et al. Heterojunction photocatalysts[J]. Advanced Materials, 2017, 29(20): 1601694. doi: 10.1002/adma.201601694
[27] 曾德彬, 杨凯, 李笑笑, 等. Ag2CO3@AgBr复合光催化剂的制备、表征及其可见光催化性能[J].有色金属科学与工程, 2018, 9(1): 51-59. http://www.xml-data.org/YSJSYKXGC/html/201801009.htm [28] LI H, TU W, ZHOU Y, et al. Z-Scheme photocatalytic systems for promoting photocatalytic performance: recent progress and future challenges[J]. Advanced Science, 2016.
[29] 于洪涛, 全燮.纳米异质结光催化材料在环境污染控制领域的研究进展[J].化学进展, 2009, 21(Z1): 406-419. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hxjz200902015 [30] TACHIBANA Y, VAYSSIERES L, DURRANT J R. Artificial photosynthesis for solar water-splitting[J]. Nature Photonics, 2012, 6(8): 511. doi: 10.1038/nphoton.2012.175
[31] LI K, SU F Y, ZHANG W D. Modification of g-C3N4 nanosheets by carbon quantum dots for highly efficient photocatalytic generation of hydrogen[J]. Applied Surface Science, 2016, 375: 110-117. doi: 10.1016/j.apsusc.2016.03.025
[32] GHOLIPOUR M R, DINH C T, BELAND F, et al. Nanocomposite heterojunctions as sunlight-driven photocatalysts for hydrogen production from water splitting[J]. Nanoscale, 2015, 7(18): 8187-8208. doi: 10.1039/C4NR07224C
[33] HU W, LIN L, ZHANG R, et al. Highly efficient photocatalytic water splitting over edge-modified phosphorene nanoribbons[J]. Journal of the American Chemical Society, 2017, 139(43): 15429-15436. doi: 10.1021/jacs.7b08474
[34] SUN S. Recent advances in hybrid Cu2O-based heterogeneous nanostructures[J]. Nanoscale, 2015, 7(25): 10850-10882. doi: 10.1039/C5NR02178B
[35] XIAO J, XIE Y, CAO H. Organic pollutants removal in wastewater by heterogeneous photocatalytic ozonation[J]. Chemosphere, 2015, 121: 1-17. doi: 10.1016/j.chemosphere.2014.10.072
[36] BARBER, JAMES. Photosynthetic energy conversion: natural and artificial[J]. Chemical Society Reviews, 2008, 38(1): 185-196. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0220784910/
[37] UMENA Y, KAWAKAMI K, SHEN J R, et al. Crystal structure of oxygen-evolving photosystem Ⅱ at a resolution of 1.9Å[J]. Nature, 2011, 473: 55-60. doi: 10.1038/nature09913
[38] LI H, TU W, ZHOU Y, et al. Z-Scheme photocatalytic systems for promoting photocatalytic performance: recent progress and future Challenges[J]. Advanced Science, 2016.
[39] LIU S, YANG M Q, Tang Z R, et al. A nanotree-like CdS/ZnO nanocomposite with spatially branched hierarchical structure for photocatalytic fine-chemical synthesis[J]. Nanoscale, 2014, 6(13): 7193. doi: 10.1039/c4nr01227e
[40] ZHANG L J, LI S, LIU B K, et al. Highly efficient CdS/WO3 photocatalysts: Z-scheme photocatalytic mechanism for their enhanced photocatalytic H2 evolution under visible light[J]. ACS Catalysis, 2014, 4(10): 3724-3729. doi: 10.1021/cs500794j
[41] 时晓羽, 李会鹏, 赵华.全固态Z-Scheme光催化材料应用于二氧化碳还原和光催化分解水研究进展[J].分子催化, 2019(4):391-396. http://d.old.wanfangdata.com.cn/Periodical/fzch201904011 [42] PATNAIK S, SWAIN G, PARIDA K. Highly efficient charge transfer through double Z-scheme mechanism by Cu promoted MoO3/g-C3N4 hybrid nanocomposite with superior electrochemical and photo catalytic performance[J]. Nanoscale, 2018: 10.1039.C7NR09049H.
[43] BARD A J. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors[J]. Journal of Photochemistry, 1979, 10(1): 59-75. doi: 10.1016/0047-2670(79)80037-4
[44] SAYAMA K, ABE R, ARAKAWA H, et al. Decomposition of water into H2 and O2 by a two-step photoexcitation reaction over a Pt-TiO2 photocatalyst in NaNO2 and Na2 CO3 aqueous solution[J]. Catalysis Communications, 2006, 7(2): 96-99. doi: 10.1016/j.catcom.2005.09.008
[45] SASAKI Y, KATO H, KUDO A.[Co(bpy)3]3+/2+ and[Co(phen)3]3+/2+ electron mediators for overall water splitting under sunlight irradiation using Z-scheme photocatalyst system[J]. Journal of the American Chemical Society, 2013, 135(14): 5441-5449. doi: 10.1021/ja400238r
[46] KATO H, SASAKI Y, SHIRAKURA N, et al. Synthesis of highly active rhodium-doped SrTiO3 powders in Z-scheme systems for visible-light-driven photocatalytic overall water splitting[J]. Journal of Materials Chemistry A, 2013, 1(39): 12327. doi: 10.1039/c3ta12803b
[47] ZHAO W, MAEDA K, ZHANG F, et al. Effect of post-treatments on the photocatalytic activity of Sm2Ti2S2O5 for the hydrogen evolution reaction[J]. Physical Chemistry Chemical Physics, 2014, 16(24): 12051. doi: 10.1039/c3cp54668c
[48] MAEDA K. Z-scheme water splitting using two different semiconductor photocatalysts[J]. ACS Catalysis, 2013, 3(7): 1486-1503. doi: 10.1021/cs4002089
[49] LI H, QUAN X, CHEN S, et al. Ferroelectric-enhanced Z-schematic electron transfer in BiVO4-BiFeO3-CuInS2 for efficient photocatalytic pollutant degradation[J]. Applied Catalysis B: Environmental, 2017, 209: 591-599. doi: 10.1016/j.apcatb.2017.03.043
[50] 陈博才, 沈洋, 魏建红, 等.基于g-C3N4的Z-型光催化体系研究进展[J].物理化学学报, 2016(6): 1371-1382. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=wlhxxb201606012 [51] CHEN F, YANG Q, LI X, et al. Hierarchical assembly of graphene-bridged Ag3PO4/Ag/BiVO4 (040) Z-scheme photocatalyst: an efficient, sustainable and heterogeneous catalyst with enhanced visible-light photoactivity towards tetracycline degradation under visible light irradiation[J]. Applied Catalysis B: Environmental, 2017, 200: 330-342. doi: 10.1016/j.apcatb.2016.07.021
[52] IWASE A, NG Y H, ISHIGURO Y, et al. Reduced graphene oxide as a solid-state electron mediator in Z-scheme photocatalytic water splitting under visible light[J]. Journal of the American Chemical Society, 2011, 133(29): 11054-11057. doi: 10.1021/ja203296z
[53] LI X, YAN X, LU X, et al. Photo-assisted selective catalytic reduction of NO by Z-scheme natural clay based photocatalyst: Insight into the effect of graphene coupling[J]. Journal of Catalysis, 2018, 357: 59-68. doi: 10.1016/j.jcat.2017.10.024
[54] LI H, TU W, ZHOU Y, et al. Z-scheme photocatalytic systems for promoting photocatalytic performance: recent progress and future challenges[J]. Advanced Science, 2016, 3(11): 1500389. doi: 10.1002/advs.201500389
[55] TADA H, MITSUI T, KIYONAGA T, et al. All-solid-state Z-scheme in CdS-Au-TiO2 three-component nanojunction system[J]. Nature Materials, 2006, 5(10): 782. doi: 10.1038/nmat1734
[56] XU Q, ZHANG L, YU J, et al. Direct Z-scheme photocatalysts: principles, synthesis, and applications[J]. Materials Today, 2018, 21(10): 1042-1063. doi: 10.1016/j.mattod.2018.04.008
[57] WANG X, LIU G, CHEN Z G, et al. Enhanced photocatalytic hydrogen evolution by prolonging the lifetime of carriers in ZnO/CdS heterostructures[J]. Chemical Communications, 2009(23): 3452-3454. doi: 10.1039/b904668b
[58] YU J, WANG S, LOW J, et al. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air[J]. Physical Chemistry Chemical Physics, 2013, 15(39): 16883-16890. doi: 10.1039/c3cp53131g
[59] ZHOU D, CHEN Z, YANG Q, et al. Facile Construction of g-C3N4 Nanosheets/TiO2 Nanotube Arrays as Z-Scheme Photocatalyst with Enhanced Visible‐Light Performance[J]. ChemCatChem, 2016, 8(19): 3064-3073. doi: 10.1002/cctc.201600828
[60] LIU X, CHEN N, LI Y, et al. A general nonaqueous sol-gel route to g-C3N4-coupling photocatalysts: the case of Z-scheme g-C3N4/TiO2 with enhanced photodegradation toward RhB under visible-light[J]. Scientific Reports, 2016, 6: 39531. doi: 10.1038/srep39531
[61] LIU J, CHENG B, YU J. A new understanding of the photocatalytic mechanism of the direct Z-scheme g-C3N4/TiO2 heterostructure[J]. Physical Chemistry Chemical Physics, 2016, 18(45): 31175-31183. doi: 10.1039/C6CP06147H
[62] BAI S, JIANG J, ZHANG Q, et al. Steering charge kinetics in photocatalysis: intersection of materials syntheses, characterization techniques and theoretical simulations[J]. Chemical Society Reviews, 2015, 44(10): 2893-2939. doi: 10.1039/C5CS00064E
[63] SHAO B, LIU X, LIU Z, et al. A novel double Z-scheme photocatalyst Ag3PO4/Bi2S3/Bi2O3 with enhanced visible-light photocatalytic performance for antibiotic degradation[J]. Chemical Engineering Journal, 2019, 368: 730-745. doi: 10.1016/j.cej.2019.03.013
[64] CONG Y, GE Y, ZHANG T, et al. Fabrication of Z-Scheme Fe2O3-MoS2-Cu2O ternary nanofilm with significantly enhanced photoelectrocatalytic performance[J]. Industrial & Engineering Chemistry Research, 2018, 57(3): 881-890. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=8531e5baebfaaa9be6cabc33f68cf3c4
[65] ZENG D, YANG K, YU C, et al. Phase transformation and microwave hydrothermal guided a novel double Z-scheme ternary vanadate heterojunction with highly efficient photocatalytic performance[J]. Applied Catalysis B: Environmental, 2018, 237: 449-463. doi: 10.1016/j.apcatb.2018.06.010
[66] HAN T, CHEN Y, TIAN G, et al. Hydrogenated TiO2/SrTiO3 porous microspheres with tunable band structure for solar-light photocatalytic H2 and O2 evolution[J]. Science China Materials, 2016, 59(12): 1003-1016. doi: 10.1007/s40843-016-5126-1
[67] NIE N, ZHANG L, FU J, et al. Self-assembled hierarchical direct Z-scheme g-C3N4/ZnO microspheres with enhanced photocatalytic CO2 reduction performance[J]. Applied Surface Science, 2018, 441: 12-22. doi: 10.1016/j.apsusc.2018.01.193
[68] 刘仁月, 吴榛, 白羽, 等.微米球光催化剂在环境净化及能源转化的研究进展[J].有色金属科学与工程, 2016, 7(6): 41-45. http://www.xml-data.org/YSJSYKXGC/html/2016060011.htm [69] 温福宇, 杨金辉, 宗旭, 等.太阳能光催化制氢研究进展[J].化学进展, 2009, 21(11): 2285-2302. http://d.old.wanfangdata.com.cn/Periodical/hndxxbzr201503006 [70] WANG Q, HISATOMI T, JIA Q, et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%[J]. Nature Materials, 2016, 15(6): 611. doi: 10.1038/nmat4589
[71] LIANG Y H, LIAO M W, MISHRA M, et al. Fabrication of Ta3N5/ZnO direct Z-scheme photocatalyst for hydrogen generation[J]. International Journal of Hydrogen Energy, 2019, 44(35): 19162-19167. doi: 10.1016/j.ijhydene.2018.07.117
[72] TIAN L, YANG X, CUI X, et al. Fabrication of dual direct Z-scheme g-C3N4/MoS2/Ag3PO4 photocatalyst and its oxygen evolution performance[J]. Applied Surface Science, 2019, 463: 9-17. doi: 10.1016/j.apsusc.2018.08.209
[73] ZHAO W, LIU J, DENG Z, et al. Facile preparation of Z-scheme CdS-Ag-TiO2 composite for the improved photocatalytic hydrogen generation activity[J]. International Journal of Hydrogen Energy, 2018, 43(39): 18232-18241. doi: 10.1016/j.ijhydene.2018.08.026
[74] WANG S, ZHU B, LIU M, et al. Direct Z-scheme ZnO/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity[J]. Applied Catalysis B: Environmental, 2019, 243: 19-26. doi: 10.1016/j.apcatb.2018.10.019
[75] YUN H J, LEE H, KIM N D, et al. A combination of two visible-light responsive photocatalysts for achieving the Z-scheme in the solid state[J]. ACS nano, 2011, 5(5): 4084-4090. doi: 10.1021/nn2006738
[76] YOU Y, WANG S, XIAO K, et al. Z-scheme g-C3N4/Bi4NbO8Cl heterojunction for enhanced photocatalytic hydrogen production[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(12): 16219-16227. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=148b544c2c70203e5c0c6c3b065781b6
[77] GUO H L, DU H, JIANG Y F, et al. Artificial photosynthetic Z-scheme photocatalyst for hydrogen evolution with high quantum efficiency[J]. The Journal of Physical Chemistry C, 2016, 121(1): 107-114. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=f7ae8ba0b61e755d36808f456979e34f
[78] XU F, ZHANG L, CHENG B, et al. Direct Z-scheme TiO2/NiS core-shell hybrid nanofibers with enhanced photocatalytic H2-production activity[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(9): 12291-12298. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=4a273e5736f7e1fad82f66d94d9f0982
[79] ZOU L, WANG H, WANG X. High efficient photodegradation and photocatalytic hydrogen production of CdS/BiVO4 heterostructure through Z-scheme process[J]. ACS Sustainable Chemistry & Engineering, 2016, 5(1): 303-309. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=a3c6fa884be5bac6f99ce02650eda551
[80] ZHANG L J, LI S, LIU B K, et al. Highly efficient CdS/WO3 photocatalysts: Z-scheme photocatalytic mechanism for their enhanced photocatalytic H2 evolution under visible light[J]. ACS Catalysis, 2014, 4(10): 3724-3729. doi: 10.1021/cs500794j
[81] YE R Q, FANG H B, ZHENG Y Z, et al. Fabrication of CoTiO3/g-C3N4 hybrid photocatalysts with enhanced H2 evolution: Z-scheme photocatalytic mechanism insight[J]. ACS Applied Materials & Interfaces, 2016, 8(22): 13879-13889.
[82] MAEDA K, LU D, DOMEN K. Solar-driven Z-scheme water splitting using modified BaZrO3-BaTaO2N solid solutions as photocatalysts[J]. ACS Catalysis, 2013, 3(5): 1026-1033. doi: 10.1021/cs400156m
[83] IWASE A, YOSHINO S, TAKAYAMA T, et al. Water splitting and CO2 reduction under visible light irradiation using Z-scheme systems consisting of metal sulfides, CoOx-loaded BiVO4, and a reduced graphene oxide electron mediator[J]. Journal of the American Chemical Society, 2016, 138(32): 10260-10264. doi: 10.1021/jacs.6b05304
[84] ARESTA M, DIBENEDETTO A, ANGELINI A. Catalysis for the valorization of exhaust carbon: from CO2 to chemicals, materials, and fuels. Technological use of CO2[J]. Chemical Reviews, 2013, 114(3): 1709-1742. doi: 10.1021/cr4002758
[85] LI X D, SUN Y F, XU J Q, et al. Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers[J]. Nature Energy, 2019, 4(8): 690-699. doi: 10.1038/s41560-019-0431-1
[86] HE Y, ZHANG L, TENG B, et al. New application of Z-scheme Ag3PO4/g-C3N4 composite in converting CO2 to fuel[J]. Environmental Science & Technology, 2014, 49(1): 649-656. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=13944253df7d34cab2c7cb85282ad989
[87] SEKIZAWA K, MAEDA K, DOMEN K, et al. Artificial Z-scheme constructed with a supramolecular metal complex and semiconductor for the photocatalytic reduction of CO2[J]. Journal of the American Chemical Society, 2013, 135(12): 4596-4599. doi: 10.1021/ja311541a
[88] DI T, ZHU B, CHENG B, et al. A direct Z-scheme g-C3N4/SnS2 photocatalyst with superior visible-light CO2 reduction performance[J]. Journal of Catalysis, 2017, 352: 532-541. doi: 10.1016/j.jcat.2017.06.006
[89] WANG J C, YAO H C, FAN Z Y, et al. Indirect Z-scheme BiOI/g-C3N4 photocatalysts with enhanced photoreduction CO2 activity under visible light irradiation[J]. ACS Applied Materials & Interfaces, 2016, 8(6): 3765-3775. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=2524476f8210c96679b03b3dff777482
[90] KUMAR A, PRAJAPATI P K, PAL U, et al. Ternary rGO/InVO4/Fe2O3 Z-scheme heterostructured photocatalyst for CO2 reduction under visible light irradiation[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(7): 8201-8211. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=9ead3174effd54f08b346223e854f1ad
[91] WANG J C, ZHANG L, FANG W X, et al. Enhanced photoreduction CO2 activity over direct Z-scheme α-Fe2O3/Cu2O heterostructures under visible light irradiation[J]. ACS Applied Materials & Interfaces, 2015, 7(16): 8631-8639. https://www.researchgate.net/publication/274643452_Enhanced_Photoreduction_CO2_Activity_over_Direct_Z-Scheme_-Fe2O3Cu2O_Heterostructures_Under_Visible_Light_Irradiation
[92] BHOSALE R, JAIN S, VINOD C P, et al. Direct Z-Scheme g-C3N4/FeWO4 nanocomposite for enhanced and selective photocatalytic CO2 reduction under visible light[J]. ACS Applied Materials & Interfaces, 2019, 11(6): 6174-6183. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=5fcd5e5b049798e23383d6024f62a00a
[93] JIN J, YU J, GUO D, et al. A Hierarchical Z-scheme CdS-WO3 photocatalyst with enhanced CO2 reduction activity[J]. Small, 2015, 11(39): 5262-5271. doi: 10.1002/smll.201500926
[94] WU F, LI X, LIU W, et al. Highly enhanced photocatalytic degradation of methylene blue over the indirect all-solid-state Z-scheme g-C3N4-RGO-TiO2 nanoheterojunctions[J]. Applied Surface Science, 2017, 405: 60-70. doi: 10.1016/j.apsusc.2017.01.285
[95] HE Y, ZHANG L, FAN M, et al. Z-scheme SnO2-x/g-C3N4composite as an efficient photocatalyst for dye degradation and photocatalytic CO2 reduction[J]. Solar Energy Materials and Solar Cells, 2015, 137: 175-184. doi: 10.1016/j.solmat.2015.01.037
[96] JO W K, SELVAM N C S. Z-scheme CdS/g-C3N4 composites with RGO as an electron mediator for efficient photocatalytic H2production and pollutant degradation[J]. Chemical Engineering Journal, 2017, 317: 913-924. doi: 10.1016/j.cej.2017.02.129
[97] XIE Z, FENG Y, WANG F, et al. Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline[J]. Applied Catalysis B: Environmental, 2018, 229: 96-104. doi: 10.1016/j.apcatb.2018.02.011
[98] LU D, WANG H, ZHAO X, et al. Highly efficient visible-light-induced photoactivity of Z-scheme g-C3N4/Ag/MoS2 ternary photocatalysts for organic pollutant degradation and production of hydrogen[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(2): 1436-1445. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=a087085deb188800b8cf23a04a8e97ea
[99] TANG H, FU Y, CHANG S, et al. Construction of Ag3PO4/Ag2MoO4 Z-scheme heterogeneous photocatalyst for the remediation of organic pollutants[J]. Chinese Journal of Catalysis, 2017, 38(2): 337-347. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201702018
[100] YANG Y, GUO W, GUO Y, et al. Fabrication of Z-scheme plasmonic photocatalyst Ag@AgBr/g-C3N4 with enhanced visible-light photocatalytic activity[J]. Journal of Hazardous Materials, 2014, 271: 150-159. doi: 10.1016/j.jhazmat.2014.02.023
[101] HE R, CHENG K, WEI Z, et al. Room-temperature in situ fabrication and enhanced photocatalytic activity of direct Z-scheme BiOI/g-C3N4 photocatalyst[J]. Applied Surface Science, 2019, 465: 964-972. doi: 10.1016/j.apsusc.2018.09.217
[102] HE R, ZHOU J, FU H, et al. Room-temperature in situ fabrication of Bi2O3/g-C3N4 direct Z-scheme photocatalyst with enhanced photocatalytic activity[J]. Applied Surface Science, 2018, 430: 273-282. doi: 10.1016/j.apsusc.2017.07.191
[103] WANG F, LI W, GU S, et al. Facile fabrication of direct Z-scheme MoS2/Bi2WO6 heterojunction photocatalyst with superior photocatalytic performance under visible light irradiation[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2017, 335: 140-148. doi: 10.1016/j.jphotochem.2016.11.026
[104] LIN H, CA J, LUO B, et al. Synthesis of novel Z-scheme AgI/Ag/AgBr composite with enhanced visible light photocatalytic activity[J]. Catalysis Communications, 2012, 21: 91-95. doi: 10.1016/j.catcom.2012.02.008
[105] LI W, CHEN J, GUO R, et al. Facile fabrication of a direct Z-scheme MoO3/Ag2CrO4 composite photocatalyst with improved visible light photocatalytic performance[J]. Journal of Materials Science: Materials in Electronics, 2017, 28(21): 15967-15979. doi: 10.1007/s10854-017-7495-0
[106] LIU H, DU C, BAI H, et al. Fabrication of plate-on-plate Z-scheme SnS2/Bi2MoO6 heterojunction photocatalysts with enhanced photocatalytic activity[J]. Journal of Materials Science, 2018, 53(15): 10743-10757. doi: 10.1007/s10853-018-2296-2
[107] UTKA A, VANAGS M, JOOST U, et al. Aqueous synthesis of Z-scheme photocatalyst powders and thin-film photoanodes from earth abundant elements[J]. Journal of Environmental Chemical Engineering, 2018, 6(2): 2606-2615. doi: 10.1016/j.jece.2018.04.003
[108] WU X F, LI H, PAN J C, et al. Designing visible-light-driven direct Z-scheme Ag2WO4/WS2 heterojunction to enhance photocatalytic activity[J]. Journal of Materials Science: Materials in Electronics, 2018, 29(17): 14874-14882. doi: 10.1007/s10854-018-9625-8
[109] QIAO Q, HUANG W Q, LI Y Y, et al. In-situ construction of 2D direct Z-scheme g-C3N4/g-C3N4 homojunction with high photocatalytic activity[J]. Journal of Materials Science, 2018, 53(23): 15882-15894. doi: 10.1007/s10853-018-2762-x
[110] DING J, DAI Z, QIN F, et al. Z-scheme BiO1-xBr/Bi2O2CO3 photocatalyst with rich oxygen vacancy as electron mediator for highly efficient degradation of antibiotics[J]. Applied Catalysis B: Environmental, 2017, 205: 281-291. doi: 10.1016/j.apcatb.2016.12.018
[111] HEZAM A, NAMRATHA K, PONNAMMA D, et al. Direct Z-scheme Cs2O-Bi2O3-ZnO heterostructures as efficient sunlight-driven photocatalysts[J]. ACS Omega, 2018, 3(9): 12260-12269. doi: 10.1021/acsomega.8b01449
[112] HUANG Z, ZENG X, LI K, et al. Z-scheme NiTiO3/g-C3N4 heterojunctions with enhanced photoelectrochemical and photocatalytic performances under visible LED light irradiation[J]. ACS Applied Materials & Interfaces, 2017, 9(47): 41120-41125. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3a828be640c4f6b56ce4fe5c460b7053
[113] LI Q, GUAN Z, WU D, et al. Z-scheme BiOCl-Au-CdS heterostructure with enhanced sunlight-driven photocatalytic activity in degrading water dyes and antibiotics[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(8): 6958-6968. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=b80fb822b8a22c5aa759d0dd5efb2a4e
[114] LI C, YU S, CHE H, et al. Fabrication of Z-scheme heterojunction by anchoring mesoporous γ-Fe2O3 nanospheres on g-C3N4 for degrading tetracycline hydrochloride in water[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(12): 16437-16447.
[115] 熊威, 葛建华, 陈羽冲, 等. g-C3N4光催化还原Cr(Ⅵ)研究进展[J].广州化工, 2018, 46(1): 12-14. doi: 10.3969/j.issn.1001-9677.2018.01.006 [116] WIEDERHOLD J G. Metal stable isotope signatures as tracers in environmental geochemistry[J]. Environmental Science & Technology, 2015, 49(5): 2606-2624. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=6f4f747a94434900d6dc26eea3fce753
[117] ZHOU Y, CHEN G, YU Y, et al. A new oxynitride-based solid state Z-scheme photocatalytic system for efficient Cr(VI) reduction and water oxidation[J]. Applied Catalysis B: Environmental, 2016, 183: 176-184. doi: 10.1016/j.apcatb.2015.10.040
[118] CHEN A, BIAN Z, XU J, et al. Simultaneous removal of Cr(VI) and phenol contaminants using Z-scheme bismuth oxyiodide/reduced graphene oxide/bismuth sulfide system under visible-light irradiation[J]. Chemosphere, 2017, 188: 659-666. doi: 10.1016/j.chemosphere.2017.09.002
[119] CHEN F, YANG Q, WANG Y, et al. Efficient construction of bismuth vanadate-based Z-scheme photocatalyst for simultaneous Cr (VI) reduction and ciprofloxacin oxidation under visible light: Kinetics, degradation pathways and mechanism[J]. Chemical Engineering Journal, 2018, 348: 157-170. doi: 10.1016/j.cej.2018.04.170
[120] YU C, CHEN F, ZENG D, et al. A facile phase transformation strategy for fabrication of novel Z-scheme ternary heterojunctions with efficient photocatalytic properties[J]. Nanoscale, 2019, 11(16): 7720-7733. doi: 10.1039/C9NR00709A
-
期刊类型引用(4)
1. 冯秀娟,王小青,张书荣,董成亮. 离子吸附型稀土原地浸矿颗粒运移与孔隙结构演变及模型构建研究现状及展望. 稀土. 2025(02): 114-127 .  百度学术
百度学术
2. 王梦园,黄诗云,刘红昌,刘洋,李京娜,聂珍媛,夏金兰,王军. 黄色黏球菌/铜绿假单胞菌-离子型稀土矿相互作用比较研究. 生物学杂志. 2024(03): 11-20+45 .  百度学术
百度学术
3. 蔡万青,周丹,秦磊,赵永红. 硫酸镁在离子型稀土中的吸附规律及形态分布研究. 有色金属科学与工程. 2024(06): 941-951 .  本站查看
本站查看
4. 程玥淞,黄玲玲,郭敏,汪实,王钧,宫清华,袁少雄. 注压对离子型稀土浸出及孔隙结构的影响. 有色金属工程. 2023(12): 51-58 .  百度学术
百度学术
其他类型引用(5)



 下载:
下载: