Preparation and application of nano-micro Ag2CO3 photocatalytic materials
-
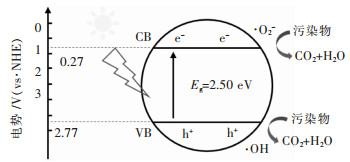
摘要: Ag2CO3是近年来发现的一种新型的可见光响应光催化剂,对甲基橙(MO)、罗丹明B(RhB)和亚甲基蓝(MB)等染料和苯酚等有机物都具有较高的光催化降解能力.然而,在光催化反应过程中,Ag2CO3晶体中的Ag+会被自身的光生电子(e-)还原形成金属Ag单质,随着反应的进行,样品的稳定性和光催化效果迅速降低.贵金属沉积、非金属掺杂和形成异质结等方法,可以使Ag2CO3的光吸收得到扩展,同时促进光生电子空穴对的分离,从而提升Ag2CO3的抗光腐蚀性能和对污染物的降解效率.通过不同的物理和化学方法调控Ag2CO3催化材料的形貌、晶粒尺寸和晶体缺陷等,可以提升其比表面积和光生电子空穴的传输效率,进而提高其光催化活性.文中归纳了近年来Ag2CO3光催化材料的研究进展,分析了Ag2CO3光催化的特点,阐述了一系列提升Ag2CO3光催化性能的方法,并对Ag2CO3光催化材料的研究进行了总结与展望.Abstract: Ag2CO3 is a new type of visible light responsive photocatalyst discovered in recent years, which with efficient photodegradation ability for methyl orange (MO), rhodamine B (RhB), methylene blue (MB) dyes and phenols. But, Ag+ in Ag2CO3 is easy to be reduced Ag by the photogenarated e-, which induced the fast photocorrosion and poor stability in the photocatalytic reaction process.The light absorption region, the stability and photocatalytic activities of Ag2CO3 semiconductor can be extended by noble metal deposition, nonmetal doping and formation of heterojunction, meanwhile, the separation of photo-generated electron-hole pairs can also be promoted. The physical properties such as morphology, crystal size and crystal defects of Ag2CO3 can be controlled by different physical and chemical methods to obtain high specific surface area, unique morphology and high separation efficiency of photo-generated electron holes and superior photocatalytic activity. The research progress of Ag2CO3 nano photocatalyst is reviewed and its photocatalytic character is analyzed. The strategies for enhancing the photocatalytic activity and stability of Ag2CO3 are summarized and discussed, and the research prospect of Ag2CO3 nano-micro photocatalyst is proposed.
-
Keywords:
- Ag2CO3 /
- morphology control /
- heterojunction /
- corrosion resistance /
- electron-hole pairs
-
0 引言
20 世纪60 年代,下向分层胶结充填采矿技术开始在我国金属矿山应用.下向分层胶结充填采矿法,由于取消了钢筋混凝土底板和钉隔离层,胶结充填体将作为下个分层采场的顶板,其强度直接影响下个分层采场的作业安全[1].
现阶段,尾砂胶结充填使用的胶凝材料主要是普通硅酸盐水泥[2].硅酸盐系列水泥的物理力学性质可以满足矿山胶结充填的大部分需求.然而水泥早期强度不高,可能会影响安全生产作业.
针对矿山特殊生产实际需求,国内外投入了大量人力、物力研究水泥的替代品.新型尾砂胶结剂正是在基于水泥的基础上,针对尾砂的物理、力学性质发展出来的一种新型硅铝基灰渣胶凝材料.该材料具有细度高(流动性、分散性能好)、水化热小、凝结时间可调等特点,可以满足矿山尾砂胶结充填的大部分要求.
目前,某铜矿采用下向进路式分层胶结充填采矿法,充填分3 期进行,充填材料为水泥和分级尾砂,浆体浓度约为70 %,一期用水泥、分级尾砂灰砂比为1∶4的浆体充填至进路高度的一半,待沉降完成后;再用水泥、分级尾砂灰砂比为1∶8 的浆体进行二期充填;最后,用全尾砂充填至接顶.根据采场安全要求及经济效益分析得出,一期用新型尾砂胶结剂、分级尾砂灰砂比为1∶6 的充填浆体,二期用新型尾砂胶结剂、分级尾砂灰砂比为1∶12 的充填浆体,替代原有水泥充填工艺.
下向分层胶结充填法回采矿体,上分层胶结充填体将作为下个分层采场的顶板,这要求充填体具有一定的抗压、抗拉强度[3-6].采用新型尾砂胶结剂替代水泥,作为分级尾砂充填体顶板的胶结剂,充填体强度将直接决定采场顶板的稳定性,从而关系到整个采场作业安全.本文将通过相关强度试验及FLAC3D 数值模拟以验证新型尾砂胶结剂胶结分级尾砂充填体是否满足采场安全作业的要求.
1 强度试验
试验中制备新型尾砂胶结剂胶结分级尾砂充填体试件采用的灰砂比为1∶6 和1∶12,42.5 级水泥胶结分级尾砂充填体试件采用灰砂比1∶4 和1∶8,其中1∶4 与1∶6 的充填体在本次试验中简称高灰砂比,1∶8 与1∶12 的充填体简称低灰砂比.本文中所涉及的强度试验均由RMT-150C 岩石力学试验系统完成.
1.1 抗压强度
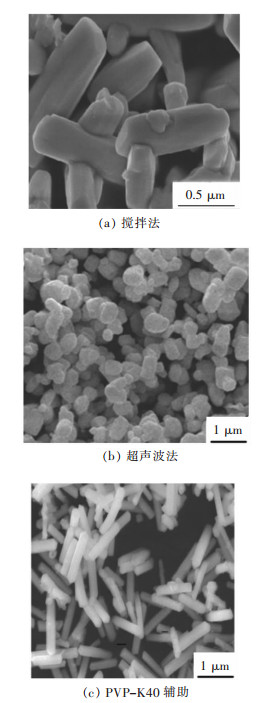
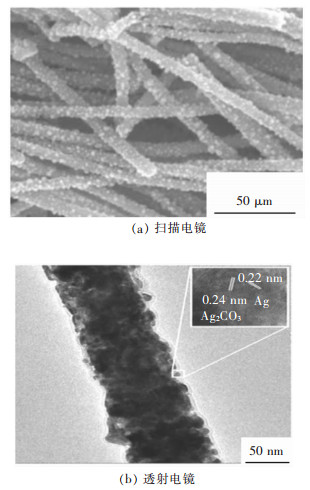
按照浆体浓度70 %,新型尾砂胶结剂与分级尾砂灰砂比为1∶6、1∶12,42.5 级水泥与分级尾砂灰砂比为1∶4、1∶8,制备φ50 mm×h100 mm 圆柱体标准试件由RMT-150C 岩石力学试验机完成单轴抗压试验[7-8],见图 1、图 2,得到了充填体不同龄期的抗压强度,结果见表 1、表 2 和图 3、图 4.
表 1 不同养护龄期,高灰砂比胶结分级尾砂充填体抗压强度试验结果 表 2 不同养护龄期,低灰砂比胶结分级尾砂充填体抗压强度试验结果
表 2 不同养护龄期,低灰砂比胶结分级尾砂充填体抗压强度试验结果
由表 1、表 2 及图 3、图 4 可知,在浆体浓度为70 %,7 d、14 d、28 d、60 d 养护龄期,灰砂比为1∶6、1∶12 的新型尾砂胶结剂胶结分级尾砂充填体的抗压强度,均高于对应灰砂比为1∶4、1∶8 的42.5 级水泥胶结分级尾砂充填体.灰砂比为1∶6 的新型尾砂胶结剂胶结分级尾砂充填体,早期(7 d 养护龄期)抗压强度为3.10 MPa, 已达到长期(28 d 养护龄期)抗压强度的61 %,而与之对应的1∶4 的42.5 级水泥胶结分级尾砂充填体早期(7 d 养护龄期)抗压强度为2.55 MPa, 仅达到长期(28 d 养护龄期)抗压强度的52 %;灰砂比为1∶12 的新型尾砂胶结剂胶结分级尾砂充填体,早期(7 d 养护龄期)抗压强度为1.18 MPa, 已达到长期(28 d 养护龄期)抗压强度的54 %,而与之对应的1∶8的42.5 级水泥胶结分级尾砂充填体早期(7 d 养护龄期)抗压强度为1.55 MPa, 仅达到长期(28 d 养护龄期)抗压强度的47 %.
1.2 抗拉强度
按照浆体浓度70 %,新型尾砂胶结剂与分级尾砂灰砂比为1∶6、1∶12,42.5 级水泥与分级尾砂灰砂比为1∶4、1∶8,制备φ50 mm×h50 mm 圆柱体标准试件.由RMT-150C 岩石力学试验机完成巴西劈裂试验[7-8],见图 5、图 6,得到了充填体不同养护龄期的抗压强度,结果见表 3、表 4 和图 7、图 8.
表 3 不同养护龄期,高灰砂比胶结分级尾砂充填体抗拉强度试验结果 表 4 不同养护龄期,低灰砂比胶结分级尾砂充填体抗拉强度试验结果
表 4 不同养护龄期,低灰砂比胶结分级尾砂充填体抗拉强度试验结果
由表 3、表 4 及图 7、图 8 可知,在浆体浓度为70 %,7 d、14 d、28 d、60 d 养护龄期,灰砂比为1∶6、1∶12 的新型尾砂胶结剂胶结分级尾砂充填体的抗拉强度,均高于对应灰砂比为1∶4、1∶8 的42.5 级水泥胶结分级尾砂充填体.灰砂比为1∶6 的新型尾砂胶结剂胶结分级尾砂充填体,早期(7 d 养护龄期)抗拉强度为0.39 MPa, 已达到长期(28 d 养护龄期)抗拉强度的65 %,而与之对应的1∶4 的42.5 级水泥胶结分级尾砂充填体早期(7 d 养护龄期)抗拉强度为0.24 MPa, 仅达到长期(28 d 养护龄期)抗拉强度的43 %;灰砂比为1∶12 的新型尾砂胶结剂胶结分级尾砂充填体,早期(7 d 养护龄期)抗拉强度为0.25 MPa, 已达到长期(28 d 养护龄期)抗拉强度的63 %,而与之对应的1∶8 的42.5 级水泥胶结分级尾砂充填体早期(7 d 养护龄期)抗拉强度为0.15 MPa, 仅达到长期(28 d 养护龄期)抗拉强度的42 %.
2 FLAC3D 模拟
采用FLAC3D (Fast Lagrangin Analysis of Continua)分别模拟用新型尾砂胶结剂、42.5 级水泥胶结分级尾砂充填后,其作为下个分层采场顶板,回采下个分层.
FLAC3D 是一个用于分析和解决工程力学问题的数值分析软件,其采用三维快速拉格朗日法分析问题,主要适用于岩石和其它材料的三维结构受力特性模拟和塑性流动分析,FLAC3D 数值软件在采矿、岩土工程界得到了广泛的应用和认可[9-11].
2.1 数值模型
由于地下局部开挖对其周围一定范围内造成影响,为提高数值模拟的准确性,整个模型的设计为进路断面尺寸的5 倍[12-14].本算例中根据某铜矿工程实际中,回采进路断面尺寸4 m×3 m(宽×高),建立数值模型.考虑进路长度远远大于进路的宽度及高度,可将本算例视为平面应变问题,进路长度设计为1 m 即可.通过分析最终确定计算模型几何尺寸44 m×1 m×33 m(长×宽×高).
本次数值模拟主要考虑充填体顶板的稳定性,故在竖直方向的网格进行了加密处理,即在竖直方向上每0.5 m 划分一个单元,水平方向每1 m 划分一个单元,整个模型的网格数目为44×1×66.模型从上至下分,依次是上覆围岩、充填体、矿体.在下向分层胶结充填采矿中,经研究发现,充填体的受力传递在3 个分层之间较为明显,4 个分层以上,充填体的受力传递影响已可忽略不计[15],所以本算例中,设计充填体由3 个分层组成.模型上边界施加0.04 MPa 的垂直应力,约束条件取两侧为限制水平方向位移的滑动支座,底部为限制垂直方向和水平方向位移的固定支座,且限制整个模型y 方向的移动.考虑到实际应用中,会在充填体中布设竖筋以增强充填体的整体性,其直径一般为16 mm, 长度为充填高度的60 %,布设网格为1.6 m×1.6 m.在本例中,用FLAC3D 的锚索单元模拟竖筋的作用.整个数值模型及网格划分见图 9.模型划分2 904 个结构单元和6 030 个节点单元.矿体、围岩、充填体及锚杆的力学、物理参数见表 5,表 6.
表 5 矿体、围岩及充填体物理力学参数 表 6 锚杆力学参数
表 6 锚杆力学参数
2.2 FLAC3D 模拟结果及分析
根据上述建立的模型,分别模拟在新型尾砂胶结剂胶结分级尾砂充填体中布设竖筋、未布设竖筋,及原有42.5 级水泥胶结分级尾砂充填,回采下个分层,得到了采场周围的塑性区分布图、最大主应力、最小主应力及垂直位移云图.
进路回采后,采场周围的塑性区分布情况见图 10.其中图 10(a)是布设竖筋,新型尾砂胶结剂胶结分级尾砂充填下进路开挖后塑性区分布情况,从图 10(a)中可以看出,仅采场的底板存在少量张拉塑性区分布;图 10(b)是未布设竖筋,新型尾砂胶结剂胶结分级尾砂充填下进路开挖后塑性区分布情况,从图 10(b)中可以看出,采场的顶板、底板都存在张拉塑性区分布;图 10(c)是布设竖筋,42.5 水泥级水泥胶结分级尾砂充填下进路开挖后塑性区分布情况,从图 10(c)中可以看出,除了采场底板存在张拉塑性区分布,充填顶板也存在剪切塑性区分布;从中可得出,在0.04 MPa 垂直应力作用下,采用新型尾砂胶结剂胶结分级尾砂充填,并布设竖筋,进路开挖后采场更稳定.
进路回采后,采场周围的最大主应力分布情况见图 11.其中图 11(a)是布设竖筋,新型尾砂胶结剂胶结分级尾砂充填下进路开挖后最大主应力分布情况,图 11(b)是未布设竖筋,新型尾砂胶结剂胶结分级尾砂充填下进路开挖后最大主应力分布情况,图 11(c)是布设竖筋,42.5 水泥级水泥胶结分级尾砂充填下进路开挖后最大主应力分布情况,从图 11 中可以看出,进路以上3 个充填分层区域也均处于受拉状态,最大应力值都为0.5 MPa, 且底板都由张拉应力控制,最大应力值分别为2.20 MPa、2.23 MPa、2.20 MPa.
进路回采后,采场周围的最小主应力分布情况见图 12.从图 12 中可以看出进路开挖后都受四周(尤其是进路两侧)拱挤压的作用,从图 12 中可以看出进路的两侧帮受压力控制,压应力集中程度较高,压应力值也最大.图 12(a)是布设竖筋,新型尾砂胶结剂胶结分级尾砂充填下采场最小主应力分布情况,最大压应力值2.39 MPa.图 12(b)是未布设竖筋,新型尾砂胶结剂胶结分级尾砂充填下采场最小主应力分布情况,最大压应力值2.38 MPa.图 12(c)是布设竖筋,42.5 水泥级水泥胶结分级尾砂充填下采场最小主应力分布情况,其应力集中与新型尾砂胶结剂胶结分级尾砂充填下采场最小主应力分布情况类似,最大压应力值2.40 MPa, 三者相差不大.
进路回采后,采场竖直方向位移分布情况见图 13.图 13(a)是布设竖筋,新型尾砂胶结剂胶结分级尾砂充填下进路开挖后的竖直方向位移分布云图,从图 13 中可以看出,充填体顶板最大沉降值为1.73 cm; 图 13(b)是未布设竖筋,新型尾砂胶结剂胶结分级尾砂充填下进路开挖后的竖直方向位移分布云图,从图 13(b)中可以看出,充填体顶板最大沉降值为2.30 cm; 图 13(c)是布设竖筋,42.5 水泥级水泥胶结分级尾砂充填下进路开挖后的竖直方向位移分布云图,从图 13(c)中可以看出,充填体顶板最大沉降值为1.81 cm.其中进路开挖对上部充填体变形扰动范围,图 13(a)、图 13(c)较图 13(b)明显更大,这是因为竖筋发挥了悬吊作用,将充填体组合成了一个整体,布置竖筋有效缓解了顶板充填体的变形,降低了顶板充填体的沉降,对提高充填体顶板稳定性具有积极地效果.为保障下个分层采场作业安全,采用新型尾砂胶结剂胶结分级尾砂充填顶板,必须布设竖筋.
通过以上塑性区分布、最大主应力、最小主应力、及垂直位移云图分析得出,在布设竖筋的情况下,新型尾砂胶结剂胶结分级尾砂充填体顶板可以满足要求.
3 结论
1) 灰砂比为1∶6 和1∶12 的新型尾砂胶结剂胶结分级尾砂充填体的7 d、14 d、28 d、60 d 养护龄期的抗压、抗拉强度分别高于对应灰砂比为1∶4 和1∶8的42.5 级水泥胶结分级尾砂充填体的抗压、抗拉强度;
2) 灰砂比为1∶6 和1∶12 的新型尾砂胶结剂胶结分级尾砂充填体,在养护龄期内的抗压、抗拉强度增长均快于对应灰砂比为1∶4 和1∶8 的42.5 级水泥胶结分级尾砂充填体的抗压、抗拉强度,灰砂比为1∶6和1∶12 的新型尾砂胶结剂胶结分级尾砂充填体的早期抗压、抗拉强度优于对应灰砂比为1∶4 和1∶8 的42.5 级水泥胶结分级尾砂充填体;
3) 在下向分层胶结采矿方法中,采用新型尾砂胶结剂胶结分级尾砂充填,必须布设竖筋,且灰砂比为1∶6 和1∶12 的新型尾砂胶结剂胶结分级尾砂替代对应灰砂比为1∶4 和1∶8 的42.5 级水泥胶结分级尾砂充填采场顶板,其稳定性可以满足要求.
-
-
[1] GUO S H, BAO J X, HU T, et al. Controllable synthesis porous Ag2CO3 nanorods for efficient photocatalysis[J]. Nanoscale Research Letters, 2015, 10(1):193-200. doi: 10.1186/s11671-015-0892-5
[2] 曹文杰, 徐俊晖, 王亚珍.石墨烯及其复合材料吸附降解有机污染物的研究进展[J].江汉大学学报(自然科学版), 2017, 45(4):298-306. http://d.old.wanfangdata.com.cn/Periodical/jhdxxb201704002 [3] LONCAREVIC D, VUKOJE I, DOSTANIC J, et al. Antimicrobial and photocatalytic abilities of Ag2CO3 nano-rods[J]. ChemistrySelect, 2017, 2(10):2931-2938. doi: 10.1002/slct.201700003
[4] 王茀学, 衣晓虹, 王崇臣, 等.一种稳定二维配位聚合物用于光催化还原Cr (Ⅵ)及降解有机污染物[J].催化学报, 2017, 38(12):2141-2149. http://www.cnki.com.cn/Article/CJFDTotal-CHUA201712023.htm [5] WOJTYLA S, BARAN T. Insight on doped ZnS and its activity towards photocatalytic removing of Cr(Ⅵ) from wastewater in the presence of organic pollutants[J]. Materials Chemistry & Physics, 2018, 212:103-112. http://www.chemeurope.com/en/publications/1170647/insight-on-doped-zns-and-its-activity-towards-photocatalytic-removing-of-cr-vi-from-wastewater-in-the-presence-of-organic-pollutants.html
[6] 高博, 刘彬, 王新, 等.半导体材料联合超声用于降解有机污染物研究进展[J].生态与农村环境学报, 2018, 34(6):481-488. http://d.old.wanfangdata.com.cn/Periodical/ncsthj201806001 [7] YU H J, SHI R, ZHAO Y X, et al. Alkali-assisted synthesis of nitrogen deficient graphitic carbon nitride with tunable band structures for efficient visible-light-driven hydrogen evolution[J]. Advanced Materials, 2017, 29(16):1605148-1605154. doi: 10.1002/adma.201605148
[8] 马小帅, 陈范云, 张萌迪, 等. G-C3N4基光催化剂的制备和应用[J].有色金属科学与工程, 2018, 9(3):42-52. http://ysjskx.paperopen.com/oa/DArticle.aspx?type=view&id=201803008 [9] 薛霜霜, 何洪波, 吴榛, 等.研磨-焙烧法制备BiOI/BiOBr异质结光催化剂及其光催化性能[J].有色金属科学与工程, 2017, 8(1):86-93. http://ysjskx.paperopen.com/oa/DArticle.aspx?type=view&id=201701015 [10] FANG X Z, SHANG Q C, WANG Y, et al. Single Pt atoms confined into a metal-organic framework for efficient photocatalysis[J]. Advanced Materials, 2018, 30(7):1705112-170519. doi: 10.1002/adma.201705112
[11] LI J D, YU C L, FANG W, et al. Preparation, characterization and photocatalytic performance of heterostructured AgCl/Bi2WO6 microspheres[J]. Chinese Journal of Catalysis, 2015, 36(7):987-993. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201507012
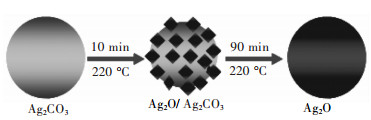
[12] 魏龙福, 余长林, 陈建钗, 等.水热法合成Ag2CO3/ZnO异质结复合光催化剂及其光催化性能[J].有色金属科学与工程, 2014, 5(1):47-53. http://ysjskx.paperopen.com/oa/DArticle.aspx?type=view&id=201401009 [13] WANG G Z, YUAN H K, CHANG J L, et al. ZnO/MoX2 (X=S, Se) composites used for visible light photocatalysis[J]. Rsc Advances, 2018, 8(20):10828-10835. doi: 10.1039/C7RA10425A
[14] LI H, LI J, AI Z H, et al. Oxygen vacancy-mediated photocatalysis of BiOCl: reactivity, selectivity, and perspectives[J]. Angewandte Chemie International Edition, 2018, 57(1):122-138. doi: 10.1002/anie.201705628
[15] 魏龙福, 余长林.石墨烯/半导体复合光催化剂的研究进展[J].有色金属科学与工程, 2013, 4(3):34-39. http://ysjskx.paperopen.com/oa/DArticle.aspx?type=view&id=201303007 [16] 樊启哲, 钟立钦, 冯庐平, 等.肖特基型光催化剂研究进展[J].材料导报, 2018, 31(9):106-111. http://d.old.wanfangdata.com.cn/Periodical/cldb201709014 [17] ZENG D B, YANG K, YU C L, et al. Phase transformation and microwave hydrothermal guided a novel double Z-scheme ternary vanadate heterojunction with highly efficient photocatalytic performance[J]. Applied Catalysis B Environmental, 2018, 237:449-463. doi: 10.1016/j.apcatb.2018.06.010
[18] WU Z, ZENG D B, LIU X Q, et al. Hierarchical δ-Bi2O3/Bi2O2CO3 composite microspheres: phase transformation fabrication, characterization and high photocatalytic performance[J]. Research on Chemical Intermediates, 2018, 44(10):5995-6010. doi: 10.1007/s11164-018-3471-4
[19] YU C L, WU Z, LIU R Y, et al. Novel fluorinated Bi2MoO6, nanocrystals for efficient photocatalytic removal of water organic pollutants under different light source illumination[J]. Applied Catalysis B Environmental, 2017, 209:1-11. doi: 10.1016/j.apcatb.2017.02.057
[20] YU C L, LIU R Y, WU Z, et al. The excellent dye-photosensitized degradation performance over hierarchical BiOCl nanostructures fabricated via a facile microwave-hydrothermal process[J]. New Journal of Chemistry, 2018, 42:137-149 doi: 10.1039/C7NJ02990J
[21] XIAO P Y, LOU J F, ZHANG H X, et al. Enhanced visible-light-driven photocatalysis from WS2 quantum dots coupled to BiOCl nanosheets: synergistic effect and mechanism insight[J]. Catalysis Science & Technology, 2018, 8(1):201-209. https://pubs.rsc.org/en/content/articlelanding/2018/cy/c7cy01784g#!
[22] 刘仁月, 吴榛, 白羽, 等.微米球光催化剂在环境净化及能源转化的研究进展[J].有色金属科学与工程, 2016, 7(6):62-72. http://ysjskx.paperopen.com/oa/DArticle.aspx?type=view&id=2016060011 [23] 白羽, 吴榛, 刘仁月, 等.花状Pt/Bi2WO6微米晶合成、表征及其高可见光催化性能[J].有色金属科学与工程, 2016, 7(2):60-66. http://ysjskx.paperopen.com/oa/DArticle.aspx?type=view&id=201602011 [24] HE H B, XUE S S, WU Z, et al. Synthesis and characterization of robust Ag2S/Ag2WO4 composite microrods with enhanced photocatalytic performance[J]. Journal of Materials Research, 2016, 31(17):2598-2607. doi: 10.1557/jmr.2016.284
[25] YU C L, WU Z, LIU R Y, et al. The effects of Gd3+ doping on the physical structure and photocatalytic performance of Bi2MoO6 nanoplate crystals[J]. Journal of Physics & Chemistry of Solids, 2016, 93:7-13. https://www.sciencedirect.com/science/article/abs/pii/S0022369716300300
[26] YU C L, WU Z, LIU R Y, et al. Novel N/Bi-BiOCl nanoplates synthesised in NH3 atmosphere and their enhanced photocatalytic activity[J]. Materials Research Innovations, 2018, 22(3):121-127. doi: 10.1080/14328917.2016.1264847
[27] 曾德彬, 杨凯, 李笑笑, 等. Ag2CO3@AgBr复合光催化剂的制备, 表征及其可见光催化性能[J].有色金属科学与工程, 2018, 9(1):51-59. http://ysjskx.paperopen.com/oa/DArticle.aspx?type=view&id=201801009 [28] 田坚, 刘珍, 魏龙福, 等.可见光驱动的核壳结构Ag2S@Ag2CO3催化剂及其对污染物的降解性能[J].有色金属科学与工程, 2017, 8(6):23-35. http://ysjskx.paperopen.com/oa/DArticle.aspx?type=view&id=2017060005 [29] PIRHASHEMI M, HABIBI A. Photosensitization of ZnO by AgBr and Ag2CO3: Nanocomposites with tandem n-n heterojunctions and highly enhanced visible-light photocatalytic activity[J]. Journal of Colloid and Interface Science, 2016, 474:103-113. https://www.sciencedirect.com/science/article/pii/S0021979716302429
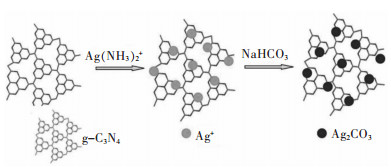
[30] YU C L, WEI L F, CHEN J C, et al. Enhancing the photocatalytic performance of commercial TiO2 crystals by coupling with trace narrow-band-gap Ag2CO3[J]. Industrial & Engineering Chemistry Research, 2014, 53(14):5759-5766. doi: 10.1021/ie404283d
[31] YU C L, WEI L F, ZHOU W Q, et al. A visible-light-driven core-shell like Ag2S@ Ag2CO3 composite photocatalyst with high performance in pollutants degradation[J]. Chemosphere, 2016, 157:250-261. doi: 10.1016/j.chemosphere.2016.05.021
[32] WANG H Q, LI J Z, HUO P W, et al. Preparation of Ag2O/Ag2CO3/MWNTs composite photocatalysts for enhancement of ciprofloxacin degradation[J]. Applied Surface Science, 2016, 366:1-8. doi: 10.1016/j.apsusc.2015.12.229
[33] LIU Y, KONG J J, YUAN J L, et al. Enhanced photocatalytic activity over flower-like sphere Ag/Ag2CO3/BiVO4 plasmonic heterojunction photocatalyst for tetracycline degradation[J]. Chemical Engineering Journal, 2018, 331:242-254. doi: 10.1016/j.cej.2017.08.114
[34] XIAO P, YUAN H Y, LIU J Q, et al. Radical mechanism of isocyanide-alkyne cycloaddition by multicatalysis of Ag2CO3, solvent, and substrate[J]. ACS Catalysis, 2015, 5(10):6177-6184. doi: 10.1021/acscatal.5b01703
[35] DONG C, WU K L, WEI X W, et al. Synthesis of graphene oxide-Ag2CO3 composites with improved photoactivity and anti-photocorrosion[J]. Crystengcomm, 2013, 16(4):730-736. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=d00fcec03ec1a3e8bc66de09a47a6e9a
[36] YOSHIKAWA M, YAMADA S, KOGA N. Phenomenological interpretation of the multistep thermal decomposition of silver carbonate to form silver metal[J]. The Journal of Physical Chemistry C, 2014, 118(15):8059-8070. doi: 10.1021/jp501407p
[37] KE J, LIU J, SUN H Q, et al. Facile assembly of Bi2O3/Bi2S3/MoS2 n-p heterojunction with layered n-Bi2O3, and p-MoS2, for enhanced photocatalytic water oxidation and pollutant degradation[J]. Applied Catalysis B Environmental, 2017, 200:47-55. doi: 10.1016/j.apcatb.2016.06.071
[38] LIU D T, LI S B, ZHANG P, et al. Efficient planar heterojunction perovskite solar cells with Li-doped compact TiO2 layer[J]. Nano Energy, 2017, 31:462-468. doi: 10.1016/j.nanoen.2016.11.028
[39] ZHU M, DENG X C, LIN X, et al. The carbon quantum dots modified ZnO/TiO2, nanotube heterojunction and its visible light photocatalysis enhancement[J]. Journal of Materials Science Materials in Electronics, 2018, 29(13):11449-11456. doi: 10.1007/s10854-018-9237-3
[40] JIN Y J, LINGHU J J, CHAI J W, et al. Defect evolution enhanced visible-light photocatalytic activity in nitrogen-doped anatase TiO2 thin films[J]. Journal of Physical Chemistry C, 2018, 122(29):16600-16606. doi: 10.1021/acs.jpcc.8b04517
[41] LI Y Y, CAO S B, ZHANG A, et al. Carbon and nitrogen Co-doped bowl-like Au/TiO2, nanostructures with tunable size for enhanced visible-light-driven photocatalysis[J]. Applied Surface Science, 2018, 445:350-358. doi: 10.1016/j.apsusc.2018.03.187
[42] WEI Q, WANG Y, QIN H Y, et al. Construction of r-GO wrapping octahedral Ag-Cu2O heterostructure for enhanced visible light photocatalytic activity[J]. Applied Catalysis B: Environmental, 2018, 227:132-144. doi: 10.1016/j.apcatb.2018.01.003
[43] WANG X T, ZHOU J Q, ZHAO S, et al. Synergistic effect of adsorption and visible-light photocatalysis for organic pollutant removal over BiVO4/carbon sphere nanocomposites[J]. Applied Surface Science, 2018, 453:394-404. doi: 10.1016/j.apsusc.2018.05.073
[44] YE M Y, ZHAO Z H, HU Z F, et al. 0D/2D heterojunctions of vanadate quantum dots/graphitic carbon nitride nanosheets for enhanced visible-light-driven photocatalysis[J]. Angewandte Chemie International Edition, 2017, 129(29):8407-8411. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=53f25d73419b8b29440c2cb658522ab7
[45] WEN X J, NIU C G, ZHANG L, et al. A novel Ag2O/CeO2 heterojunction photocatalysts for photocatalytic degradation of enrofloxacin: possible degradation pathways, mineralization activity and an in depth mechanism insight[J]. Applied Catalysis B Environmental, 2018, 221:701-714. doi: 10.1016/j.apcatb.2017.09.060
[46] HE K L, XIE J, LI M L, et al. In situ one-pot fabrication of g-C3N4 nanosheets/NiS cocatalyst heterojunction with intimate interfaces for efficient visible light photocatalytic H2 generation[J]. Applied Surface Science, 2018, 430:208-217. doi: 10.1016/j.apsusc.2017.08.191
[47] LOW J X, YU J G, JARONIEC M, et al. Heterojunction photocatalysts[J]. Advanced Materials, 2017, 29(20):1601694-1601713. doi: 10.1002/adma.v29.20
[48] XU C W, LIU Y Y, HUANG B B, et al. Preparation, characterization, and photocatalytic properties of silver carbonate[J]. Applied Surface Science, 2011, 257(20):8732-8736. http://cn.bing.com/academic/profile?id=8a0d7a9586933671f393aa2059224209&encoded=0&v=paper_preview&mkt=zh-cn
[49] ZHOU L, LIANG L Y, TALIFU D, et al. Sonochemical fabrication of Ag2CO3 nanomaterial and influencing factors on photocatalytic properties[C]//IOP Conference Series: Materials Science and Engineering. IOP Publishing, 2017, 167(1): 012032.
[50] YU N, DONG R H, LIU J J, et al. Synthesis of Ag/Ag2CO3 heterostructures with high length-diameter ratios for excellent photoactivity and anti-photocorrosion[J]. Rsc Advances, 2016, 106: 103938-103943. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=c51586565827c69a9e8688955b463659
[51] DAI G, LI S, LIU S, et al. Improved Photocatalytic Activity and Stability of Nano-sized Ag/Ag2CO3 Plasmonic Photocatalyst by Surface Modification of Fe(Ⅲ) Nanocluster[J]. Journal of the Chinese Chemical Society, 2015, 62(11): 944-950. doi: 10.1002/jccs.v62.11
[52] TIAN J, LIU R Y, LIU Z, et al. Boosting the photocatalytic performance of Ag2CO3, crystals in phenol degradation via, coupling with trace N-CQDs[J]. Chinese Journal of Catalysis, 2017, 38(12):1999-2008. doi: 10.1016/S1872-2067(17)62926-7
[53] LIU S Q, WANG S, DAI G P, et al. Enhanced Visible-Light Photocatalytic Activity and Stability of Nano-Sized Ag2CO3 Combined with Carbon Nanotubes[J]. Acta Physico-Chimica Sinica, 2014, 30(11): 2121-2126.
[54] DONG H J, CHEN G, SUN J X, et al. Highly-effective photocatalytic properties and interfacial transfer efficiencies of charge carriers for the novel Ag2CO3/AgX heterojunctions achieved by surface modification[J]. Dalton Transactions, 2014, 43(19):7282-7289. doi: 10.1039/C4DT00058G
[55] LI J J, YANG W L, NING J Q, et al. Rapid formation of AgnX (X= S, Cl, PO4, C2O4) nanotubes via an acid-etching anion exchange reaction[J]. Nanoscale, 2014, 6(11): 5612-5615. doi: 10.1039/C4NR00364K
[56] YU C L, LI G, KUMAR S, et al. Phase transformation synthesis of novel Ag2O/Ag2CO3 heterostructures with high visible light efficiency in photocatalytic degradation of pollutants[J]. Advanced Materials, 2014, 26(6):892-898. doi: 10.1002/adma.v26.6
[57] ZHAO X L, SU Y C, QI X D, et al. A facile method to prepare novel Ag2O/Ag2CO3 three-dimensional hollow hierarchical structures and their water purification function[J]. Acs Sustainable Chemistry & Engineering, 2017, 5(7):6148-6158. doi: 10.1021/acssuschemeng.7b01040
[58] FA W J, WANG P, YUE B, et al. Ag3PO4/Ag2CO3 p-n heterojunction composites with enhanced photocatalytic activity under visible light[J]. Chinese Journal of Catalysis, 2015, 36(12):2186-2193. doi: 10.1016/S1872-2067(15)61004-X
[59] FANG S S, DING C Y, LIANG Q, et al. In-situ precipitation synthesis of novel BiOCl/Ag2CO3, hybrids with highly efficient visible-light-driven photocatalytic activity[J]. Journal of Alloys and Compounds, 2016, 684:230-236. doi: 10.1016/j.jallcom.2016.05.168
[60] WANG J, DONG C, JIANG B B, et al. Preparation of visible light-driven Ag2CO3/BiOBr composite photocatalysts with universal degradation abilities[J]. Materials Letters, 2014, 131(12):108-111. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=b1a3bbdeeac7fa62c59251d6589f302f
[61] WANG N, SHI L, YAO L Z, et al. Highly improved visible-light-induced photocatalytic performance over BiOI/Ag2CO3 heterojunctions[J]. Rsc Advances, 2018, 8(1):537-546. https://pubs.rsc.org/en/content/articlelanding/2018/ra/c7ra10423e#!divAbstract
[62] LI T T, HU X L, LIU C C, et al. Efficient photocatalytic degradation of organic dyes and reaction mechanism with Ag2CO3/Bi2O2CO3 photocatalyst under visible light irradiation[J]. Journal of Molecular Catalysis A: Chemical, 2016, 425:124-135. doi: 10.1016/j.molcata.2016.10.001
[63] 刘仁月, 吴榛, 白羽, 等. Ag2CO3/BiVO4复合微米片光催化剂的制备, 表征及光催化机理[J].无机化学学报, 2017, 33(3):519-527. http://www.cnki.com.cn/Article/CJFDTotal-WJHX201703020.htm [64] DAI G P, LIU S Q, LIANG Y, et al. Fabrication of a nano-sized Ag2CO3/reduced graphene oxide photocatalyst with enhanced visible-light photocatalytic activity and stability[J]. Rsc Advances, 2014, 65(4):34226-34231. https://pubs.rsc.org/en/content/articlelanding/2014/ra/c4ra04792c#!divAbstract
[65] LI J D, WEI L F, YU C L, et al. Preparation and characterization of graphene oxide/Ag2CO3 photocatalyst and its visible light photocatalytic activity[J]. Applied Surface Science, 2015, 358:168-174. doi: 10.1016/j.apsusc.2015.07.007
[66] XU H, SONG Y X, SONG Y H, et al. Synthesis and characterization of g-C3N4/Ag2CO3 with enhanced visible-light photocatalytic activity for the degradation of organic pollutants[J]. RSC Advances, 2014, 4(65):34539-34547. https://pubs.rsc.org/en/content/articlelanding/2014/ra/c4ra03443k#!divAbstract
[67] TONDA S, KUMAR S, SHANKER V. In situ growth strategy for highly efficient Ag2CO3/g-C3N4 hetero/nanojunctions with enhanced photocatalytic activity under sunlight irradiation[J]. Journal of Environmental Chemical Engineering, 2015, 3(2):852-861. doi: 10.1016/j.jece.2015.03.021
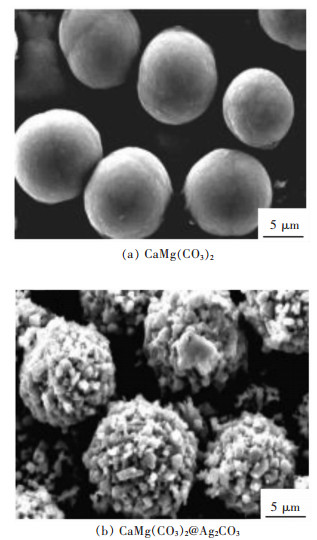
[68] TIAN J, WU Z, LIU Z, et al. Low-cost and efficient visible-light-driven CaMg(CO3)2@Ag2CO3 microspheres fabricated via an ion exchange route[J]. Chinese Journal of Catalysis, 2017, 38(11):1899-1908. doi: 10.1016/S1872-2067(17)62924-3
[69] PANTHI G, PARK S J, KIM T W, et al. Electrospun composite nanofibers of polyacrylonitrile and Ag2CO3 nanoparticles for visible light photocatalysis and antibacterial applications[J]. Journal of Materials Science, 2015, 50(13):4477-4485. doi: 10.1007/s10853-015-8995-z
[70] CHEN J, DING N W, LI Z F, et al. Organic cathode material for lithium ion battery[J]. Progress in Chemistry, 2015, 27 (9): 1291-1301. http://d.old.wanfangdata.com.cn/Periodical/gfzxb201704008




 下载:
下载: