A study on the synthesis and electrochemical properties of CuFe2O4 cubes as anode material for lithium-ion batteries
-
摘要: 金属氧化物材料具有多倍于商业石墨负极的理论容量,但此类材料在储锂过程中会出现体积膨胀,导致活性物质粉化脱落,影响锂离子电池的循环寿命。以金属有机框架(MOFs)普鲁士蓝立方体为自牺牲模板合成了空心CuFe2O4立方颗粒,并将其作为锂离子电池的负极材料。CuFe2O4立方块的粒径范围在300~500 nm之间,壳层厚度为40 nm。电化学测试表明CuFe2O4立方颗粒在200 mA/g电流密度下循环200次后放电容量仍能达到742.4 mAh/g,出色的性能得益于颗粒的中空结构能够有效缓解因储锂而产生的体积膨胀,从而延长锂离子电池的循环寿命。Abstract: Metal oxide materials have many times of the theoretical capacity of commercial graphite anode, but the volume expansion of metal oxide materials in the lithium storage process leads to the pulverization and shedding of active materials, which affects the cycle life of lithium-ion batteries. In this paper, hollow CuFe2O4 cubic particles were synthesized by hydrothermal method using metal organic frameworks (MOFs) Prussian blue micro-cubes as self-sacrificial templates, and were used as anode materials for lithium ion batteries. The particle size of CuFe2O4 cubes is between 300 nm and 500 nm, and the thickness of CuFe2O4 shell is about 40 nm. After 200 cycles at a current density of 200 mA/g, the discharge capacity of CuFe2O4 cubes still reaches 742.4 mAh/g. The excellent performance is attributed to the hollow structure of CuFe2O4 cubes, which could effectively alleviate the volume expansion caused by lithium storage and prolong the cycle life of lithium-ion batteries.
-
Keywords:
- copper ferrite /
- metal organic framework /
- lithium-ion battery /
- anode material
-
在过去的几十年里,锂离子电池在便携式电脑、混合动力汽车和其他可充电环保电子设备中得到了蓬勃发展,随着经济的发展,社会对下一代具有更高能量密度和更长的循环寿命的锂离子电池的需求日益增长,刺激了新的电极材料的发展[1-3]。目前,商用负极材料主要是理论比容量仅为372 mAh/g的石墨,因此研发出更高比容量的电池负极材料显得尤为关键[4-5]。过渡金属氧化物MFe2O4(M=Ni, Zn, Cu等)的立方尖晶石结构有利于锂离子的存储,在此晶体结构中Fe3+占据了四面体位置,而M2+占据紧密堆积的O2-离子的八面体间隙位置[6-7]。特别是CuFe2O4,由于其具有低成本、高丰度和环境友好性等优点,以及895 mAh/g的高理论容量,被众多研究者认为极有前途的是锂离子电池负极材料之一[8]。
尽管高理论容量的尖晶石型CuFe2O4因具有众多的优点而受到了研究者的青睐,但仍存在一些缺点限制了其应用,其中最主要的问题是CuFe2O4的循环稳定性较差,这是因为随着锂离子的嵌入,负极材料会出现明显的体积膨胀,而储能过程的体积变化将恶化电池性能,如电极材料的破碎、粉化,电解液的分解和过度消耗等,从而降低了锂离子电池的循环寿命[9]。除此以外,受制于较低的电导率,CuFe2O4的倍率性能也表现欠佳。为了解决以上问题,众多研究者将精力集中在材料的纳米化和特殊结构构筑上,颗粒纳米化后电子和锂离子的扩散路径将大大缩短,倍率性能将得到增强,而一些特殊的颗粒结构能够有效缓解储锂造成的体积膨胀,提高材料的电化学稳定性[10-12]。
金属有机骨架(MOFs)是一类由金属中心/簇与桥联的有机配体组装而成的新型材料,MOFs通常能形成元素分布均匀的规则颗粒并包含许多相互连接的通道[13-14]。MOFs可以作为自牺牲模板剂,通过煅烧合成各类大小均一、具有多孔外壳的氧化物颗粒。普鲁士蓝,是一种典型的MOFs材料[15]。在本项工作中,首先利用纳米晶体定向聚合,制备了规则形貌的普鲁士蓝立方颗粒,再将普鲁士蓝颗粒分散于醋酸铜溶液中,以离子替换的方式将其转化成Cu/Fe双金属普鲁士蓝立方颗粒,经过煅烧之后,最终获得了中空结构的CuFe2O4立方颗粒。所得的CuFe2O4立方颗粒表现出优良的电化学性能,在200 mA/g电流密度下循环200次后放电容量仍能达到729.1 mAh/g。
1 实验部分
1.1 材料合成
1.1.1 普鲁士蓝(Fe4(Fe(CN)6)3)的制备
称取20 g聚乙烯吡咯烷酮(K30,平均分子量58 000)溶于250 mL(0.1 mol/L)HCl溶液中,磁力搅拌30 min直至PVP完全溶解,再加入0.6 g的K4Fe(CN)6·3H2O,磁力搅拌30 min直至澄清的淡黄色溶液,取出磁转子,用保鲜膜封闭后80 ℃油浴20 h,自然冷却后抽滤,再用乙醇水溶液(30%乙醇)清洗离心3遍,真空烘箱60 ℃干燥12 h得到普鲁士蓝立方块。
1.1.2 CuFe2O4立方颗粒的制备
分别取0.1 g普鲁士蓝立方块和0.064 g的Cu(CH3COO)2·H2O分散在20 mL乙醇中,在30 ℃下磁力搅拌2 h,经离心水洗4遍后,在真空烘箱中70 ℃干燥24 h得到前驱体,将前驱体置于空气气氛的马弗炉中,以2 ℃/min的升温速率升温至500 ℃,保温2 h后自然冷却,即可得到CuFe2O4立方颗粒。
1.2 材料的表征
合成的CuFe2O4立方颗粒采用日本理学公司的Rigaku Mini Flex600 X’pert型X射线衍射仪(XRD, Cu靶,λ=0.154 05 nm)进行物相分析,用Tescan MIRA3型场发射扫描电子显微镜(FESEM)和JEM-2100型高分辨率透射电镜(HRTEM)观察样品的形貌和结构。
1.3 材料的电化学测试
工作电极是以合成的CuFe2O4、导电碳(Super P)和黏结剂(PVDF)按照质量比7:2:1混合而成,以金属锂片为对电极,Celgard 2400聚丙烯微孔膜为隔膜,以浓度为1 mol/L的LiPF6溶解在EC+DMC+DEC(V:V:V=1:1:1)的有机溶液为电解液,在氩气氛手套箱里组装成CR2016型扣式电池。在LAND-V34电池性能测试系统上进行恒电流充放电实验和倍率性能测试,电压范围为1.0~3.0 V。
2 结果与讨论
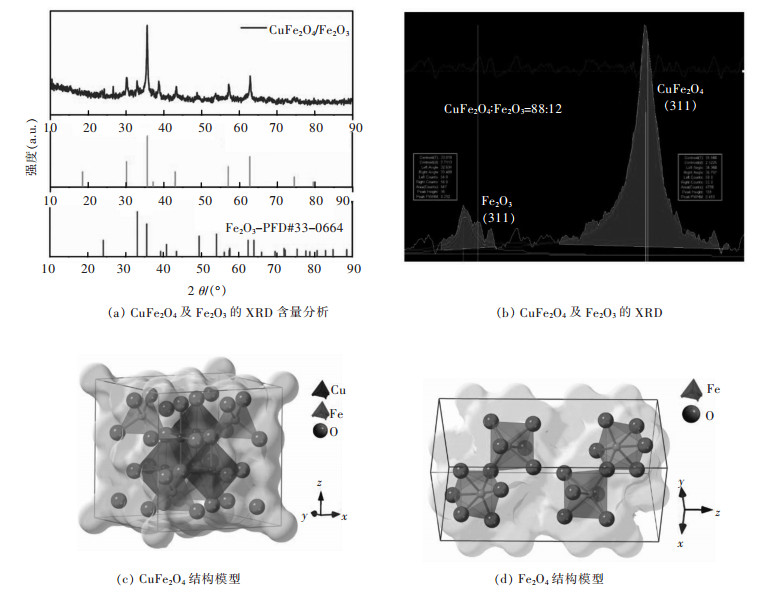
图 1(a)所示是CuFe2O4立方颗粒的XRD谱图。从图 1(a)中可以看出,所得材料在30.1°、35.6°、37.2°、43.0°、57.1°和62.8°的衍射峰能够准确对应上立方结构的CuFe2O4(PDF# 25-0283)的(220)、(311)、(222)、(400)、(511)和(440)晶面。值得注意的是,在33.1°和43.3°观测到2个衍射峰,分别对应了Fe2O3的(104)和(202)晶面,表明所得的材料含有少量的Fe2O3存在。从XRD的分析结果来看,用Cu离子替代部分Fe离子,结合热处理,成功制备了CuFe2O4材料。由于Cu元素替换的Fe元素的数量有限,导致部分Fe元素富余,热解后多余的Fe元素生成了少量Fe2O3杂相。如图 1(b),对CuFe2O4和Fe2O3的特征峰拟合可知CuFe2O4与Fe2O3的摩尔比为88:12,进一步对XRD数据拟合表明所得CuFe2O4属于空间群为Fd3m的立方晶系,如图 1(c),其晶格参数a=0.837 nm,晶胞体积为0.586 376 nm3。而Fe2O3相则属于空间群R3C三方晶系,如图 1(d),其晶格参数a=5.038,c=1.37 720 nm,晶胞体积为0.302 722 nm3。Fe2O3同样具有较强的储锂能力,Fe2O3相与CuFe2O4相的两相结构所产生的丰富的相界,可能会产生大量的电子快速传递和离子扩散的活性位点和外部缺陷,能够有效增强电极材料的扩散动力学。
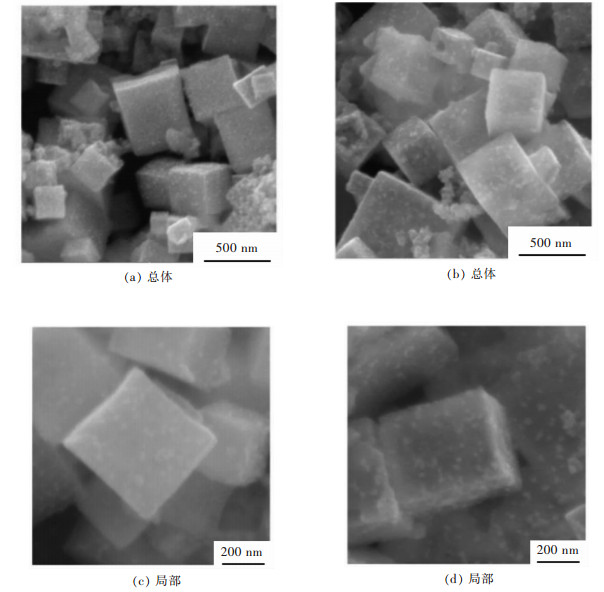
图 2所示是所制备CuFe2O4的扫描电镜图,从图 2(a)中可以看到所得的CuFe2O4形貌呈现立方块状,颗粒粒径分布范围在300~500 nm之间,颗粒表面较为粗糙,表现为疏松多孔的形态,这种结构往往具有较大的比表面积,有利于电解液的渗透,增大反应的活化面积,从而缩短锂离子的扩散路径,增强充放电比容量。在图 2(b)中可以清楚地看到有一个破损的CuFe2O4立方颗粒存在大孔洞,间接说明了立方颗粒内部是中空结构。
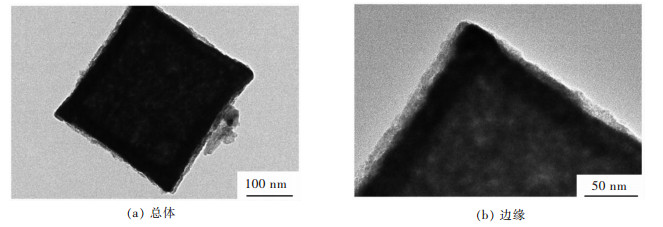
为了了解CuFe2O4立方颗粒的内部结构,采用透射电镜对材料进行了观测。从图 3(a)中可以看出立方颗粒确实呈现了中空结构,在图 3(b)中颗粒的边框厚度较为均匀,大约为40 nm。中空的结构能够非常有效的缓解储锂造成的体积膨胀应力,有助于提升材料的稳定性,避免过早出现粉化等电极恶化现象。透射电镜的结果进一步说明了中空结构的CuFe2O4立方颗粒被成功合成。这种中空结构的形成主要是由于前驱体固体颗粒在热分解过程中产生的大量的质量/体积损失,特别是在非平衡条件下,引起颗粒表面和内部的非均质收缩,从而形成明显的空洞结构[16-17]。
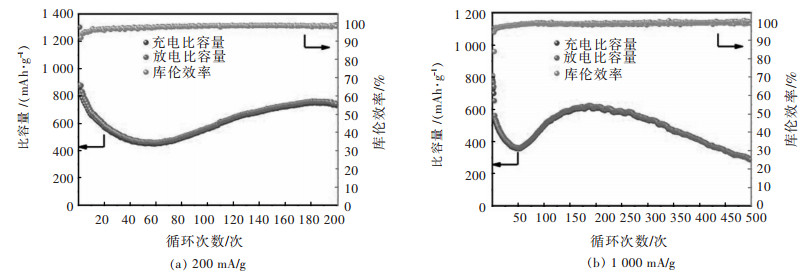
图 4所示为CuFe2O4立方颗粒作为锂离子电池电极材料的循环稳定性图。在图 4(a)中,电池的电流密度为200 mA/g,首次放电容量达到1 302.9 mAh/g,库伦效率为66.18%,这是由于首次放电过程会生成固体电解质界面膜(SEI膜),此过程为不可逆反应,导致部分的Li+被锁死[18]。电池200次的循环曲线呈现了先减小后增大,最后平稳的趋势。前60次循环电池容量逐渐下降的原因是由于循环初期,电极表面结构降解和重排与活性物质的聚合物凝胶膜的形成。60次循环后容量开始爬升则可以归结于聚合物凝胶膜开始产生赝电容效应,聚合物凝胶状薄膜可以通过所谓的“赝电容型行为”存储多余的Li+离子[19-21]。因此,容量在随后的周期中逐渐增加促进了Li+的存储。当赝电容效应趋于稳定后,电池的可逆容量也不再发生明显变化,经过200次循环后,CuFe2O4电极仍能释放出742.4 mAh/g的容量,对比第2次循环,容量保持率为84.7%。在图 4(b)中,在1 000 mA/g的高电流密度循环测试时,CuFe2O4电极表现出相似的容量变化趋势,但是经过200次循环后,电极上的活性材料难以保存结构稳定,可逆容量逐步下降,在500次循环后,放电容量仍有286.9 mAh/g。
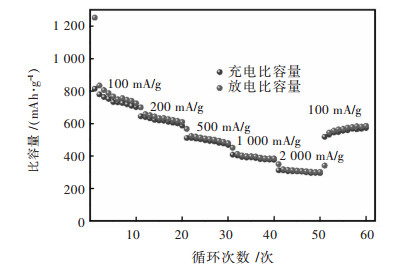
除了良好的循环稳定性,优秀的倍率性能对于电极材料来说也非常重要,图 5所示为CuFe2O4立方颗粒在100、200、500、1 000 mA/g和2 000 mA/g电流密度下的倍率性能图。CuFe2O4立方颗粒在100、200、500、1 000 mA/g和2 000 mA/g电流密度下平均放电容量分别为816.4、639.7、400.8、312.8 mAh/g,电流密度重新下降到100 mA/g后,平均放电容量恢复到547.5 mAh/g。结果表明,在大电流密度范围内,制备的材料具有良好的倍率性能,即使是在2 000 mA/g的高电流密度下相比于石墨负极具有较大的容量优势。
3 结论
以普鲁士蓝MOFs立方块为自牺牲模板,通过热处理制备了中空结构的CuFe2O4立方颗粒。颗粒粒径在300~500 nm之间,壳层厚度为40 nm。当CuFe2O4立方颗粒被用作锂离子电池负极材料时,其表现出良好的循环稳定性和倍率性能。过200次循环后,CuFe2O4电极仍能释放出742.4 mAh/g的容量,在1 000 mA/g的高电流密度下循环500次后,放电容量仍有286.9 mAh/g,良好的电化学性能得益于CuFe2O4立方颗粒疏松多孔的形态,增强了电解液的渗透,中空的内部结构则为储锂过程提供了充足的空间。
-
-
[1] 段建峰, 钟盛文, 曾敏. 20Ah富锂锰动力电池的性能研究[J].有色金属科学与工程, 2013, 4(2): 37-40. http://www.xml-data.org/YSJSYKXGC/html/201302008.htm [2] 李俊莉, 黄文进, 杨润芳, 等. Mn掺杂Co0.9Mn0.1P/RGO复合电极材料的合成及其电化学性能[J].江西冶金, 2020, 40(1): 22-26. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=jxyj202001005 [3] DENG X, LI S, WANG J, et al. Nitrogen-doped zinc/cobalt mixed oxide micro-/nanospheres for high-rate lithium-ion battery anode[J]. Journal of Materials Research, 2019, 34(18): 1-8. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=S0884291419002589
[4] LIU B, ZHANG Q, JIN Z, et al. Uniform pomegranate-like nanoclusters organized by ultrafine transition metal oxide@nitrogen-doped carbon subunits with enhanced lithium storage properties[J]. Advanced Energy Materials, 2018, 8(7): 14178-1418. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=0d55570240836cdb342f926e897b4cbc
[5] JIANG P, LIU Q, SUN X P. NiP2 nanosheet arrays supported on carbon cloth: An efficient 3D hydrogen evolution cathode in both acidic and alkaline solutions[J]. Nanoscale, 2014(6): 13440-13445. https://pubs.rsc.org/en/content/articlelanding/2014/nr/c4nr04866k
[6] LIU J, XIAO J, ZENG X, et al. Combustion synthesized macroporous structure MFe2O4 (M= Zn, Co) as anode materials with excellent electrochemical performance for lithium ion batteries[J]. Journal of Alloys & Compounds, 2017, 699: 401-407. https://www.researchgate.net/publication/255749778_Highly_ordered_mesoporous_NiO_anode_material_for_lithium_ion_batteries_with_an_excellent_electrochemical_performance
[7] LIU J, WANG R, ZHONG X, et al. Li and Na storage behavior of MgFe2O4 nanoparticles as anode materials for lithium ion and sodium ion batteries[J]. International Journal of Electrochemical Science, 2019, 14(1): 1725-1732.
[8] JIN L, QIU Y, DENG H, et al. Hollow CuFe2O4 spheres encapsulated in carbon shells as an anode material for rechargeable lithium-ion batteries[J]. Electrochimica Acta, 2011, 56(25): 9127-9132. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=7e66cc9d617e7e87943ca1324eb983c8
[9] ZHAO Y, LI X, YAN B, et al. Recent developments and understanding of novel mixed transition-metal oxides as anodes in lithium ion batteries[J]. Advanced Energy Materials, 2016, 6(8):1-19. doi: 10.1002/aenm.201502175
[10] LUO L, CUI R, QIAO H, et al. High lithium electroactivity of electrospun CuFe2O4 nanofibers as anode material for lithium-ion batteries[J]. Electrochim Acta, 2014, 144: 85-91. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=c23471506ef1f865e76a69aead74a06c
[11] KALISELVAN R, KALASISELVI N, AUGUSTIN C O, et al. CuFe2O4/SnO2 nanocomposites as anodes for Li-ion batteries[J]. J Power Sources, 2006, 157(1): 522-527.
[12] DINGY, YANG Y, SHAO H. Synthesis and characterization of nanostructured CuFe2O4 anode material for lithium ion battery[J]. Solid State Ionics, 2012, 217(11): 27-33. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=2373f948eb4298a7db8bdd95f9fa828d
[13] 刘嘉铭, 卢彦华, 王苏敏, 等.以MOFs为模板制备TMO/C复合材料在锂离子电池中的应用[J].江西冶金, 2019, 39(4): 12-16. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=jxyj201904003 [14] PENG H J, HAO G X, CHU Z H, et al. Mesoporous Mn3O4/C microspheres fabricated from MOF template as advanced lithium-ion battery anode[J]. Crystal Growth & Design, 2017, 17(11): 5881-5886. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=69a4f25727fd7d57b03a80dc9a15f7a6
[15] ZHANG L, WU H B, LOU X W. Metal-organic-frameworks-derived general formation of hollow structures with high complexity[J]. Journal of the American Chemical Society, 2013, 135(29): 10664-10672. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=93e2a8477d015012d4eaea6b92a7cfeb
[16] SUN D, TANG Y, YE D, et al. Tuning the morphologies of MnO/C hybrids by space constraint assembly of Mn-MOFs for high performance Li-ion batteries[J]. ACS Applied Materials & Interfaces, 2017, 9(6): 5254-5262. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=878ef2b0e86798f9c0f9506e202b8407
[17] WU L, WANG Z, LONG Y, et al. Multishelled NixCo3-xO4 hollow microspheres derived from bimetal-organic frameworks as anode materials for high-performance lithium-ion batteries[J]. Small, 2017, 13(17): 1604270. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=a0047d8e4b5ae870582c847646857f48
[18] ZHOU L, ZHAO D, LOU X. Double-shelled CoMn2O4 hollow microcubes as high-capacity anodes for lithium-ion batteries[J]. Advanced Materials, 2012, 24(6):745-748. doi: 10.1093-icb-icr011/
[19] WANG J, ZHANG Q, LI X, et al. Smart construction of three-dimensional hierarchical tubular transition metal oxide core/shell heterostructures with high-capacity and long-cycle-life lithium storage[J]. Nano Energy, 2015, (12): 437-446. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=b640fc3b403e72b89ded753b65ad4c77
[20] XIAO J, XU G, SUN S, et al. MFe2O4 and MFe@Oxide Core-Shell nanoparticles anchored on N-doped graphene sheets for synergistically enhancing lithium storage performance and electrocatalytic activity for oxygen reduction reactions[J]. Particle & Particle Systems Characterization, 2013, 30(10): 893-904.
[21] MAO J, HOU X, WANG X, et al. Corncob-shaped ZnFe2O4/C nanostructures for improved anode rate and cycle performance in lithium-ion batteries[J]. RSC Advances, 2015, 5(40): 31807-31814. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=c95f71dcbdfe58537fb1287c8cb06347
-
期刊类型引用(6)
1. 侯宏英,贾彦鹏,李俊凯,兰建,陈方淑. 石墨烯生产废液中双球状碳酸锰的提取及其电化学储锂性能. 有色金属科学与工程. 2024(01): 8-14 .  本站查看
本站查看
2. 刘力,杨天辉,周曦,孟冉浩. 氢化物对Mg_2Ni基合金储氢性能的影响. 有色金属科学与工程. 2023(06): 825-832 .  本站查看
本站查看
3. 胡海燕,武源波,刘益峰,唐瑞仁,吴雄伟,肖遥. 基于铝氧键稳定的隧道型钠离子电池正极材料. 有色金属科学与工程. 2022(02): 59-66 .  本站查看
本站查看
4. 张露,黄彬琪,王艳阳,龙腾威,刘嘉铭. 分级结构MoO_2/C微球作为高性能锂离子电池负极材料研究. 江西冶金. 2022(05): 31-35 .  百度学术
百度学术
5. 文敏,徐子其,张克,李轩,胡君辉,罗虹,尹艳红. 氧化钨/碳纳米管膜复合负极的制备及其储锂性能. 有色金属科学与工程. 2021(04): 58-65 .  本站查看
本站查看
6. 李基铭,覃慧,刘嘉铭. 水热法制备V_2O_5作为高性能锂离子电池正极材料. 有色金属(冶炼部分). 2021(11): 79-84 .  百度学术
百度学术
其他类型引用(0)



 下载:
下载: