Research progress in the design, fabrication and application of Z-scheme heterojunction photocatalysts
-
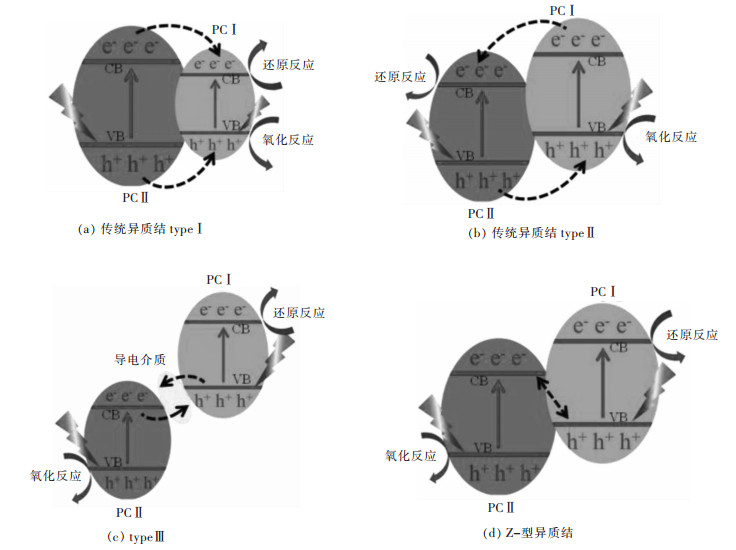
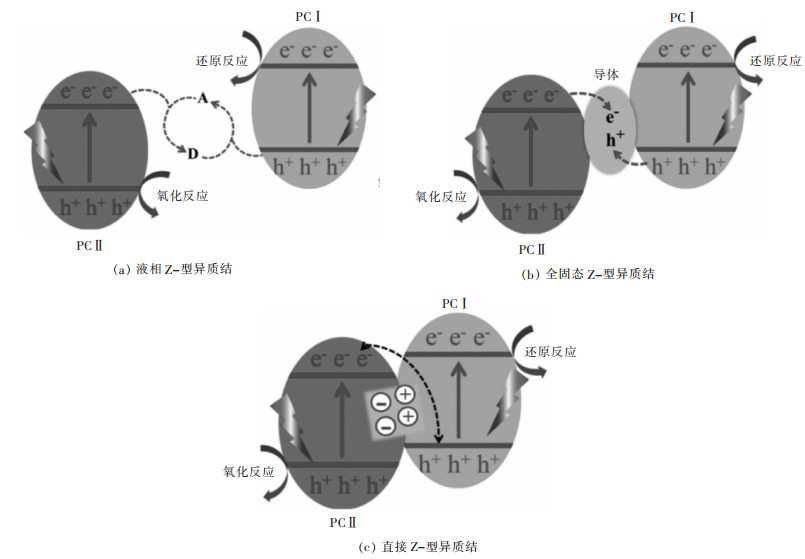
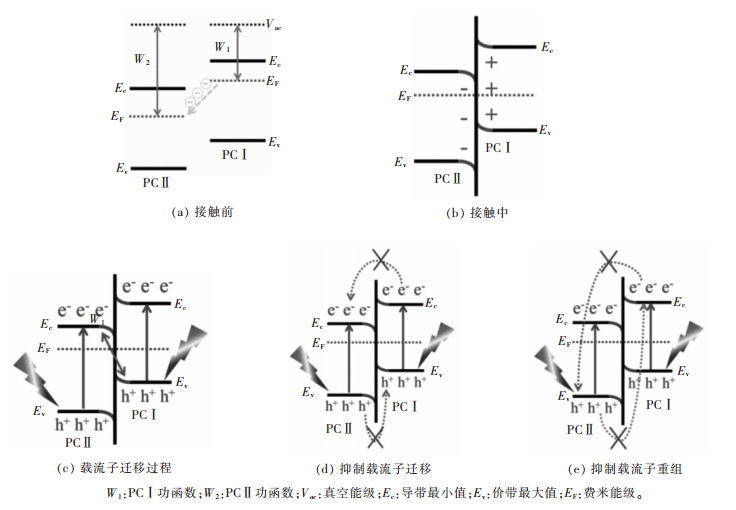
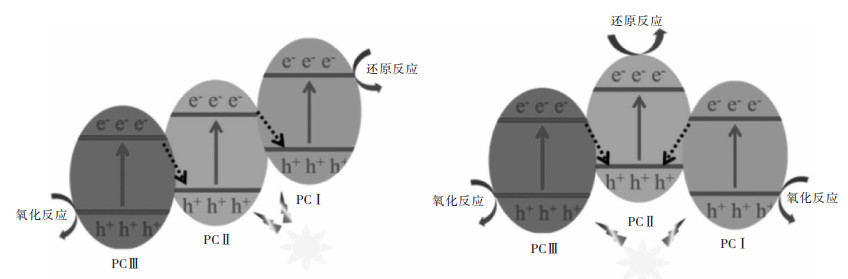
摘要: 传统异质结具有扩大光响应范围、促进载流子分离的优点,但存在氧化-还原能力不够的问题。Z-型异质结是根据自然界植物光合作用模拟的人工光合作用而提出的,相对于单一光催化剂与传统异质结光催化剂具有能有效分离电子空穴对、减少复合几率、保留强氧化-还原活性位点、扩大光响应范围、提高光催化活性等优点。文中综述了近年来,液相Z-型异质结光催化剂、全固态Z-型异质结光催化剂、直接Z-型异质结光催化剂的反应机理、构建方法与在光解水产氢、CO2还原、有机物的降解、水中重金属离子的还原等应用方面的研究进展。并对比几类Z-型异质结光催化剂的特点,提出了Z-型光催化体系发展的未来挑战和前景。Abstract: Traditional heterojunction boasts such advantages as enlargement in optical response range and facilitation in carrier separation. However, its redox ability is insufficient. Z-scheme heterojunction is put forward based on artificial photosynthesis, a simulation of plant photosynthesis in nature. With respect to the single photocatalyst and traditional heterojunction, Z-scheme heterojunction has distinct advantages, e.g. the effective separation of electron-hole pairs, reduced recombination of photo-induced carrier, strong redox active site, expanded light response range, high photocatalytic activity, etc. In this paper, the reaction mechanism, construction method and application of liquid phase Z-scheme heterojunction, all-solid-state Z-scheme heterojunction and direct Z-scheme heterojunction are reviewed. Finally, the characteristics of several types of Z-scheme heterojunction were briefly summarized and compared, and the challenges and prospects for the development of Z-scheme photocatalytic systems are proposed.
-
稀土Yb不仅对铝、镁等合金材料的力学、耐腐蚀性能的改善作用非常显著,还能与其他金属形成功能合金。开发Yb的功能合金材料,拓宽其应用领域的前景非常广阔[1-3]。目前,Yb的单质金属生产主要是通过真空镧热还原Yb2O3获得,产能低、作业不连续、成本高。同时混熔法制备Yb合金,烧损率高且易偏析,而熔盐电解法直接制备稀土金属合金能够克服热还原和混熔法的不足,提高稀土合金制备的效率[4-5]。前期研究表明采用LiF-CaF2-Yb2O3体系,Ni作为自耗阴极能制备出Ni-Yb合金,具有良好的应用前景[6]。为进一步改善电解工艺参数、降低能耗、提高电解效率,需要全面系统地研究LiF-CaF2-Yb2O3体系的物理化学性质。其中,表面张力是熔盐的一种界面性质,也是熔盐重要的物理参数之一,对界面反应以及熔盐电解过程均有较大的影响,直接关系电解过程中阳极效应以及金属产物的氧化过程[7-8]。对熔盐表面张力进行深入探讨,可为熔盐中质点间的相互作用力、熔盐电解机理、熔盐离子结构的研究提供关键数据,从而为电解工艺参数的选择提供理论依据。为此,本文采用拉筒法[9]对Yb2O3溶解度范围内的LiF-CaF2-Yb2O3体系的黏度进行测量,并通过数学模型对Ni-Yb合金表面张力进行分析和计算,从而为优化电解工艺参数提供依据,同时,为深入研究和分析LiF-CaF2-Yb2O3体系的结构及其电解机理提供必要的基础数据。
1 实验部分
分析纯CaF2、LiF、Yb2O3在423 K下烘干48 h;光谱纯石墨坩埚经乙醇清洗后,在353 K下烘干24 h。表面张力测量系统如图1所示,主要由精密电子天平与钨测头通过钢丝连接组成,当垂直的圆筒状测头与液体接触时,液体的表面张力对测头产生向下的拉力,通过测量液体表面上的圆筒测头拉离液体表面时的最大拉力(Fmax)以及圆筒周长,然后通过式(1)计算熔体的表面张力。测量流程包括:①钨测头(Φ15 mm)通过钢丝悬挂在精准电子天平上,调整电子天平和测头的位置使测头在炉膛中心处且侧头平稳没有倾斜;②通过测量高纯水在293 K下的表面张力值和在不同温度下的熔融NaCl的表面张力来校准实验装置;③校正结束后,将干燥脱水且混匀后的待测样品放入坩埚,然后利用仪器自带的高温电阻炉对样品坩埚进行加热至实验温度,并恒温20 min后,确保测头位置与校正实验时的位置相同,运行表面张力测量程序对待测样品进行表面张力测量。
(1) 式(1)中:σ为表面张力数值,单位mN/m;r为长度数值,单位m。
2 结果与讨论
2.1 LiF-CaF2-Yb2O3熔盐体系的表面张力研究
2.1.1 LiF-CaF2体系表面张力模型及数据评估
LU 等[10]及LIAO等[11]研究表明Santos方程[12]能用于二元熔盐体系表面张力的预测,且能够描述二元体系的表面张力及其表面相与体相组成的关系。通过拉筒实验测量值对Santos方程预测LiF-CaF2表面张力的适用性进行评估。Santos预测二元混合熔盐体系表面张力的模型如式(2)所示,AQRA 等[13]、HARA等[14]及MARCUS[15]研究报道的纯组元LiF和CaF2的标准表面张力值如表1所列。
表 1 纯组元LiF与CaF2的标准表面张力值Table 1. Standard surface tension values of pure components LiF and CaF2物质 表面张力/(mN/m) KT,i/GPa-1 LiF γ(LiF)=373.2-0.109T 0.093 CaF2 γ(CaF2)=459.0-0.095 6T 0.064 (2) 式(2)中:
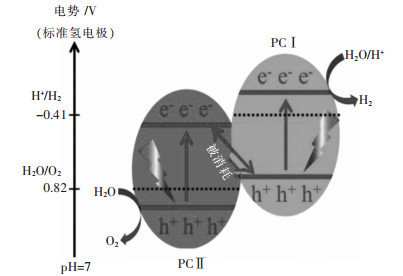
、 分别为体相A、B的摩尔分数;σid为理想纯组元的表面张力数值,单位N/m;σA、σB分别为纯组元A、B的表面张力数值,单位N/m;VA、VB分别为纯组元A、B的摩尔体积数值,单位m3/mol; 、 分别为恒温条件下纯组元A、B摩尔表面积与压力的偏导数;R为气体常数(8.314 J/K/mol);T为绝对温度数值,单位K;KT,i为组元i的等温压缩系数数值,单位Pa-1;N0为阿伏伽德罗常数(6.02×1023 mol-1);Mi为组元i的摩尔质量数值,单位kg/mol;ρi为组元i的密度数值,单位kg/m3。 图2(a)中实验测量获得的表面张力数据表明,随着温度的升高,表面张力均可近似为呈线性降低,其中,LiF-CaF2共晶体系表面张力与温度呈线性关系如式(3)所示。因为熔盐体内部及表面离子或分子间的动能增加,间距增大,离子或分子间相互吸引力减小,分子或离子的相互作用也弱化,导致表面张力值降低。根据图2(a)中数据对比可知:Santos方程计算值与不同组分LiF-CaF2体系表面张力测量值的最大估算误差为6.55%,表明Santos模型能够较好地预测LiF-CaF2表面张力。进一步利用Santos模型分析LiF-CaF2体系表面相与体相组成关系,可以看出,表面张力值随着CaF2在表面相组成的增加而增大,温度升高导致CaF2在表面相的组成减少,CaF2相对于LiF不易与在熔体表面层表面相聚集,推测与CaF2在熔体中的结构形式有关。
![]() 图 2 LiF-CaF2熔盐体系表面张力(a)实验值与Santos模型计算值比较和(b) CaF2在表面相与体相含量关系Figure 2. (a) Comparison between the experimental values and the related values calculated by the Santos model and (b) relationship between the CaF2 content in the surface phase and bulk phase of the surface tension of the LiF-CaF2 molten salt system
图 2 LiF-CaF2熔盐体系表面张力(a)实验值与Santos模型计算值比较和(b) CaF2在表面相与体相含量关系Figure 2. (a) Comparison between the experimental values and the related values calculated by the Santos model and (b) relationship between the CaF2 content in the surface phase and bulk phase of the surface tension of the LiF-CaF2 molten salt system(3) 2.1.2 LiF-CaF2-Yb2O3体系表面张力变化规律
廖春发等[16]研究表明在1 173~1 523 K温度范围内,(LiF-CaF2)etu体系中Yb2O3溶解度低于2%(质量分数),因此,图3(a)中为一定Yb2O3含量下 (LiF-CaF2)etu -Yb2O3体系表面张力随温度的变化规律,可以看出,在1 173~1 523 K范围内, 一定Yb2O3含量的(LiF-CaF2)etu -Yb2O3体系表面张力随着温度的升高而降低,原因是温度的升高致使熔体表面层离子或团簇具备较高的动能和较低的解离能,阴阳离子之间的相互吸引作用减弱。图3(b)为特定温度下(LiF-CaF2)etu-Yb2O3体系表面张力随Yb2O3含量的变化规律,可以看出,表面张力与Yb2O3的溶解度之间密切相关。当Yb2O3的质量分数为0~2%时,Yb2O3在LiF-CaF2体系内被充分溶解,体系的表面张力在Yb2O3的质量分数为1%时达到最高值。随着Yb2O3的质量分数进一步增加,达到过饱和状态,体系的表面张力出现减小,分析原因认为Yb2O3的加入会导致(LiF-CaF2)etu体系的离子构成形式发生变化,从而使表面层和体相离子团簇构成形式发生变化。由于(LiF-CaF2)etu体系中Yb2O3的溶解度较小,(LiF-CaF2)etu体系中主体结构不会发生根本性变化,但可以确定在Yb2O3的溶解量为0~2%(质量分数)时,体系内部会产生吸引作用力强的离子团簇,使其表面张力增加。随着Yb2O3含量的增加,体系表面层中离子结构发生变化,削弱了表面层离子或团簇的吸引作用,导致表面张力下降。拟合图3(b)中数据可以得到LiF-CaF2-Yb2O3熔盐体系表面张力的回归方程式(4)。
(4) 2.2 Ni-Yb合金的表面张力
综合LiF-CaF2-Yb2O3体系和Ni-Yb合金表面张力变化规律,表明随电解温度的升高,LiF-CaF2-Yb2O3熔盐体系的表面张力减小,但仅在220~260 mN/m较小范围内变化。Yb2O3在LiF-CaF2-Yb2O3体系中的含量引起的张力变化范围同样不显著,在1 173~1 523 K温度区间表面张力波动范围小于20 mN/m。因此,Ni-Yb合金的表面张力值随组分和温度的变化决定合金与LiF-CaF2-Yb2O3体系的分离。
Butler方程[17]如式(5)所示,该方程经过完善后被广泛应用于二元及三元合金内的表面张力的预测[18-19],经对多种合金体系的验证,预测值与实验值较吻合。利用Butler模型,结合Yb、Ni纯金属的标准物理参数[20],见表2所列,得到图4所示高于Ni-Yb合金熔点100~600 K和Yb摩尔百分含量0~100%范围内的表面张力曲面预测值。 同时,图4中给出了LiF-CaF2-Yb2O3体系在1 173~1 523 K温度区间以及Yb2O3质量分数在0~4%范围内的表面张力曲面预测值,当合金中Yb的摩尔分数低于10%时, 合金的表面张力变化梯度大, 在600~ 1 700 mN/m迅速增大。当Yb的摩尔分数高于10%时,合金的表面张力变化梯度小,在200~600 mN/m范围内缓慢减小。对比LiF-CaF2-Yb2O3体系的张力曲面,当合金中Yb的摩尔分数低于10%时,合金与熔盐的表面张力差值范围在340~1 480 mN/m间波动,远高于熔盐体系。合金与熔盐体系润湿性差,液态合金易于团聚。合金中Yb的摩尔分数高于10%时,合金与熔盐的表面张力差值仅在20~340 mN/m小范围内波动,合金与熔盐体系润湿性好,液态合金不易团聚。总体上, 在温度1 173~1 523 K范围内的LiF-CaF2-Yb2O3体系下,熔盐体系的黏度相对稳定,Yb摩尔分数高于10%的Ni-Yb合金产物与LiF-CaF2-Yb2O3熔盐表面张力差值较小,润湿性更好,有利于收集。
表 2 纯金属Ni、Yb的标准物理参数Table 2. Standard physical parameters for the pure metals Ni and Yb组元 表面张力(σ)/(mN/m) Ni 1 834-0.376×(T-1 455) Yb 320-0.102×(T-824) (5) 式(5)中:
=0.75,σ和σi 为溶体和纯组分i的表面张力数值,单位mN/m;R为气体常数(8.314 J/mol/K);T为绝对温度数值,单位K;XiS和XiB分别为组分i在表面相和体相中的摩尔分数;Si为组分i纯物质的单层表面积数值,单位m2; 和 分别为表面相和体相中组分i的偏摩尔吉布斯自由能数值,单位J/mol;ZS和ZB分别是表面相和体相的配位数; No为阿伏伽德罗常数(6.02×1023 mol-1);Mi为组分i的摩尔质量数值,单位kg;ρi为密度数值,单位kg/m3。 3 结 论
1)在1 173~1 523 K范围内,随着温度的升高,LiF-CaF2体系的表面张力呈线性降低。Santos方程能较好预测LiF-CaF2体系二元熔盐体系表面张力和描述表面相与体相组成的关系;在1 173~1 523 K范围内,LiF-CaF2-Yb2O3体系表面张力随温度的升高而降低;在Yb2O3的质量分数为1%~4%范围内,体系的表面张力先增后减,在质量分数为1%时达到最高。
2)当高于熔点200~600 K范围内,液态Ni-Yb合金中Yb的摩尔分数低于10%时,其表面张力在600~1 700 mN/m迅速增大。当Yb的摩尔分数高于10%时,合金的表面张力在200~600 mN/m范围内缓慢减小。温度为1 173~1 523 K范围内的LiF-CaF2-Yb2O3体系的表面张力在220~260 mN/m较小范围内波动,相对稳定,与Yb摩尔分数高于10%的Ni-Yb合金表面张力差值较小,润湿性更好。
-
表 1 Z-型异质结构建方法
Table 1 Construction method of Z-scheme heterojunction

表 2 用于光解水产氢产氧的Z-型异质结光催化剂
Table 2 Hydrogen and oxygen production over Z-scheme heterojunction photocatalysts

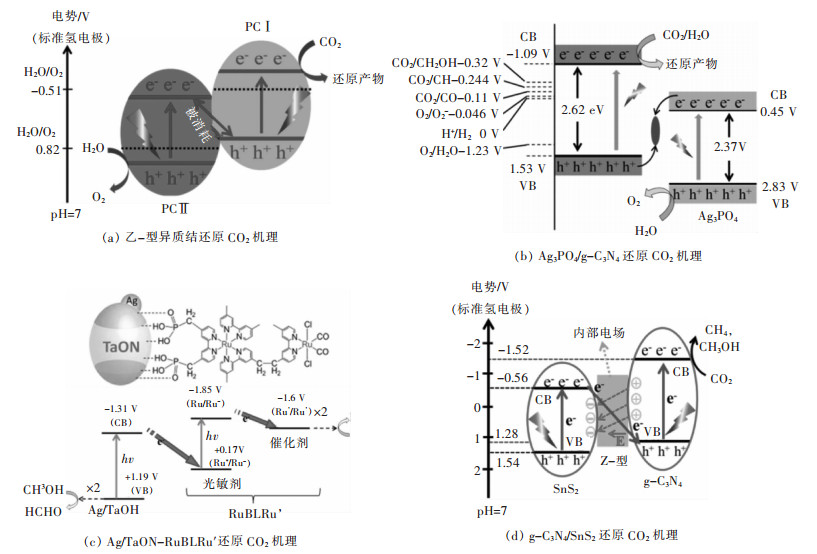
表 3 CO2还原产物及电势
Table 3 CO2 reduction products and potential

表 4 用于CO2还原的Z-型异质结光催化剂
Table 4 Reduction of CO2 over Z-scheme heterojunction photocatalysts

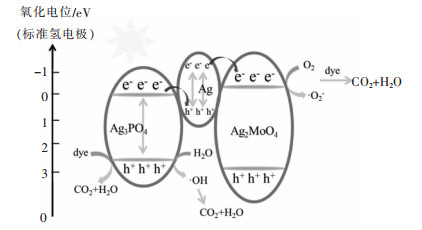
表 5 用于降解有机污染物的Z-型异质结光催化剂
Table 5 Degradation of organic pollutants over Z-scheme heterojunction photocatalysts

-
[1] BAHRUDIN N N, NAWI M A. Immobilized titanium dioxide/powdered activated carbon system for the photocatalytic adsorptive removal of phenol[J]. Korean Journal of Chemical Engineering, 2018, 35(7): 1532-1541.2. doi: 10.1007/s11814-018-0062-4
[2] MURRAY J, KING D. Climate policy: Oil's tipping point has passed[J]. Nature, 2012, 481(7382): 433. doi: 10.1038/481433a
[3] KONG L, MU X, FAN0 X, et al. Site-selected N vacancy of g-C3N4 for photocatalysis and physical mechanism[J]. Applied Materials Today, 2018, 13: 329-338. doi: 10.1016/j.apmt.2018.10.003
[4] MENG F, LIU Y, WANG J, et al. Temperature dependent photocatalysis of g-C3N4, TiO2 and ZnO: Differences in photoactive mechanism[J]. Journal of Colloid and Interface Science, 2018, 532: 321-330. doi: 10.1016/j.jcis.2018.07.131
[5] FUJISHIMA A, HONDA K. Electrochemical photolysis of water at a semiconductor electrode[J]. Nature, 1972, 238(5358): 37-38. doi: 10.1038/238037a0
[6] YU C, HE H, FAN Q, et al. Novel B-doped BiOCl nanosheets with exposed (001) facets and photocatalytic mechanism of enhanced degradation efficiency for organic pollutants[J]. Science of the Total Environment, 2019, 694: 133727. doi: 10.1016/j.scitotenv.2019.133727
[7] YU C, HE H, LIU X, et al. Novel SiO2 nanoparticle-decorated BiOCl nanosheets exhibiting high photocatalytic performances for the removal of organic pollutants[J]. Chinese Journal of Catalysis, 2019, 40(8): 1212-1221. doi: 10.1016/S1872-2067(19)63359-0
[8] YU C, WU Z, LIU R, et al. Novel fluorinated Bi2MoO6 nanocrystals for efficient photocatalytic removal of water organic pollutants under different light source illumination[J]. Applied Catalysis B: Environmental, 2017, 209: 1-11. doi: 10.1016/j.apcatb.2017.02.057
[9] YANG K, LI X, YU C, et al. Review on heterophase/homophase junctions for efficient photocatalysis: The case of phase transition construction[J]. Chinese Journal of Catalysis, 2019, 40(6): 796-818. doi: 10.1016/S1872-2067(19)63290-0
[10] YU C, ZHOU W, ZHU L, et al. Integrating plasmonic Au nanorods with dendritic like α-Bi2O3/Bi2O2CO3 heterostructures for superior visible-light-driven photocatalysis[J]. Applied Catalysis B: Environmental, 2016, 184: 1-11. doi: 10.1016/j.apcatb.2015.11.026
[11] TIAN J, WU Z, LIU Z, et al. Low-cost and efficient visible-light-driven CaMg(CO3)2@Ag2CO3 microspheres fabricated via an ion exchange route[J]. Chinese Journal of Catalysis, 2017, 38(11): 1899-1908. doi: 10.1016/S1872-2067(17)62924-3
[12] PICHAT P. A brief survey of the practicality of using photocatalysis to purify the ambient air (indoors or outdoors) or air effluents[J]. Applied Catalysis B: Environmental, 2019, 245: 770-776. doi: 10.1016/j.apcatb.2018.12.027
[13] REN H, KOSHY P, CHEN W F, et al. Photocatalytic materials and technologies for air purification[J]. Journal of Hazardous Materials, 2017, 325: 340-366. doi: 10.1016/j.jhazmat.2016.08.072
[14] CHA B J, SAQLAIN S, SEO H O, et al. Hydrophilic surface modification of TiO2 to produce a highly sustainable photocatalyst for outdoor air purification[J]. Applied Surface Science, 2019, 479: 31-38. doi: 10.1016/j.apsusc.2019.01.261
[15] FANG Y, MA Y, ZHENG M, et al. Metal-organic frameworks for solar energy conversion by photoredox catalysis[J]. Coordination Chemistry Reviews, 2018, 373: 83-115. doi: 10.1016/j.ccr.2017.09.013
[16] JIN X, YE L, XIE H, et al. Bismuth-rich bismuth oxyhalides for environmental and energy photocatalysis[J]. Coordination Chemistry Reviews, 2017, 349: 84-101. doi: 10.1016/j.ccr.2017.08.010
[17] PHURUANGRAT A, SIRI S, WADBUA P, et al. Microwave-assisted synthesis, photocatalysis and antibacterial activity of Ag nanoparticles supported on ZnO flowers[J]. Journal of Physics and Chemistry of Solids, 2019, 126: 170-177. doi: 10.1016/j.jpcs.2018.11.007
[18] YIN H, CHEN X, LI G, et al. Sub-lethal photocatalysis bactericidal technology cause longer persistence of antibiotic-resistance mutant and plasmid through the mechanism of reduced fitness cost[J]. Applied Catalysis B: Environmental, 2019, 245: 698-705. doi: 10.1016/j.apcatb.2019.01.041
[19] LEE M, SHAHBAZ H M, KIM J U, et al. Efficacy of UV-TiO2 photocatalysis technology for inactivation of Escherichia coli K12 on the surface of blueberries and a model agar matrix and the influence of surface characteristics[J]. Food Microbiology, 2018, 76: 526-532. doi: 10.1016/j.fm.2018.07.015
[20] YAO C, YUAN A, ZHANG H, et al. Facile surface modification of textiles with photocatalytic carbon nitride nanosheets and the excellent performance for self-cleaning and degradation of gaseous formaldehyde[J]. Journal of Colloid and Interface Science, 2019, 533: 144-153. doi: 10.1016/j.jcis.2018.08.058
[21] BANERJEE S, DIONYSIOU D D, PILLAI S C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis[J]. Applied Catalysis B: Environmental, 2015, 176: 396-428. https://www.sciencedirect.com/science/article/pii/S0926337315001794
[22] FAN Y, ZHOU J, ZHANG J, et al. Photocatalysis and self-cleaning from g-C3N4 coated cotton fabrics under sunlight irradiation[J]. Chemical Physics Letters, 2018, 699: 146-154. doi: 10.1016/j.cplett.2018.03.048
[23] NAUFAL B, ULLATTIL S G, PERIYAT P. A dual function nanocrystalline TiO2 platform for solar photocatalysis and self cleaning application[J]. Solar Energy, 2017, 155: 1380-1388. doi: 10.1016/j.solener.2017.08.005
[24] ZHOU P, YU, JARONIEC M. All-solid-state Z-scheme photocatalytic systems[J]. Advanced Materials, 2014, 26(29): 4920-4935. doi: 10.1002/adma.201400288
[25] QI K, CHENG B, YU J, et al. A review on TiO2-based Z-scheme photocatalysts[J]. Chinese Journal of Catalysis, 2017, 38(12): 1936-1955. doi: 10.1016/S1872-2067(17)62962-0
[26] LOW J, YU J, JARONIEC M, et al. Heterojunction photocatalysts[J]. Advanced Materials, 2017, 29(20): 1601694. doi: 10.1002/adma.201601694
[27] 曾德彬, 杨凯, 李笑笑, 等. Ag2CO3@AgBr复合光催化剂的制备、表征及其可见光催化性能[J].有色金属科学与工程, 2018, 9(1): 51-59. http://www.xml-data.org/YSJSYKXGC/html/201801009.htm [28] LI H, TU W, ZHOU Y, et al. Z-Scheme photocatalytic systems for promoting photocatalytic performance: recent progress and future challenges[J]. Advanced Science, 2016.
[29] 于洪涛, 全燮.纳米异质结光催化材料在环境污染控制领域的研究进展[J].化学进展, 2009, 21(Z1): 406-419. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hxjz200902015 [30] TACHIBANA Y, VAYSSIERES L, DURRANT J R. Artificial photosynthesis for solar water-splitting[J]. Nature Photonics, 2012, 6(8): 511. doi: 10.1038/nphoton.2012.175
[31] LI K, SU F Y, ZHANG W D. Modification of g-C3N4 nanosheets by carbon quantum dots for highly efficient photocatalytic generation of hydrogen[J]. Applied Surface Science, 2016, 375: 110-117. doi: 10.1016/j.apsusc.2016.03.025
[32] GHOLIPOUR M R, DINH C T, BELAND F, et al. Nanocomposite heterojunctions as sunlight-driven photocatalysts for hydrogen production from water splitting[J]. Nanoscale, 2015, 7(18): 8187-8208. doi: 10.1039/C4NR07224C
[33] HU W, LIN L, ZHANG R, et al. Highly efficient photocatalytic water splitting over edge-modified phosphorene nanoribbons[J]. Journal of the American Chemical Society, 2017, 139(43): 15429-15436. doi: 10.1021/jacs.7b08474
[34] SUN S. Recent advances in hybrid Cu2O-based heterogeneous nanostructures[J]. Nanoscale, 2015, 7(25): 10850-10882. doi: 10.1039/C5NR02178B
[35] XIAO J, XIE Y, CAO H. Organic pollutants removal in wastewater by heterogeneous photocatalytic ozonation[J]. Chemosphere, 2015, 121: 1-17. doi: 10.1016/j.chemosphere.2014.10.072
[36] BARBER, JAMES. Photosynthetic energy conversion: natural and artificial[J]. Chemical Society Reviews, 2008, 38(1): 185-196. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0220784910/
[37] UMENA Y, KAWAKAMI K, SHEN J R, et al. Crystal structure of oxygen-evolving photosystem Ⅱ at a resolution of 1.9Å[J]. Nature, 2011, 473: 55-60. doi: 10.1038/nature09913
[38] LI H, TU W, ZHOU Y, et al. Z-Scheme photocatalytic systems for promoting photocatalytic performance: recent progress and future Challenges[J]. Advanced Science, 2016.
[39] LIU S, YANG M Q, Tang Z R, et al. A nanotree-like CdS/ZnO nanocomposite with spatially branched hierarchical structure for photocatalytic fine-chemical synthesis[J]. Nanoscale, 2014, 6(13): 7193. doi: 10.1039/c4nr01227e
[40] ZHANG L J, LI S, LIU B K, et al. Highly efficient CdS/WO3 photocatalysts: Z-scheme photocatalytic mechanism for their enhanced photocatalytic H2 evolution under visible light[J]. ACS Catalysis, 2014, 4(10): 3724-3729. doi: 10.1021/cs500794j
[41] 时晓羽, 李会鹏, 赵华.全固态Z-Scheme光催化材料应用于二氧化碳还原和光催化分解水研究进展[J].分子催化, 2019(4):391-396. http://d.old.wanfangdata.com.cn/Periodical/fzch201904011 [42] PATNAIK S, SWAIN G, PARIDA K. Highly efficient charge transfer through double Z-scheme mechanism by Cu promoted MoO3/g-C3N4 hybrid nanocomposite with superior electrochemical and photo catalytic performance[J]. Nanoscale, 2018: 10.1039.C7NR09049H.
[43] BARD A J. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors[J]. Journal of Photochemistry, 1979, 10(1): 59-75. doi: 10.1016/0047-2670(79)80037-4
[44] SAYAMA K, ABE R, ARAKAWA H, et al. Decomposition of water into H2 and O2 by a two-step photoexcitation reaction over a Pt-TiO2 photocatalyst in NaNO2 and Na2 CO3 aqueous solution[J]. Catalysis Communications, 2006, 7(2): 96-99. doi: 10.1016/j.catcom.2005.09.008
[45] SASAKI Y, KATO H, KUDO A.[Co(bpy)3]3+/2+ and[Co(phen)3]3+/2+ electron mediators for overall water splitting under sunlight irradiation using Z-scheme photocatalyst system[J]. Journal of the American Chemical Society, 2013, 135(14): 5441-5449. doi: 10.1021/ja400238r
[46] KATO H, SASAKI Y, SHIRAKURA N, et al. Synthesis of highly active rhodium-doped SrTiO3 powders in Z-scheme systems for visible-light-driven photocatalytic overall water splitting[J]. Journal of Materials Chemistry A, 2013, 1(39): 12327. doi: 10.1039/c3ta12803b
[47] ZHAO W, MAEDA K, ZHANG F, et al. Effect of post-treatments on the photocatalytic activity of Sm2Ti2S2O5 for the hydrogen evolution reaction[J]. Physical Chemistry Chemical Physics, 2014, 16(24): 12051. doi: 10.1039/c3cp54668c
[48] MAEDA K. Z-scheme water splitting using two different semiconductor photocatalysts[J]. ACS Catalysis, 2013, 3(7): 1486-1503. doi: 10.1021/cs4002089
[49] LI H, QUAN X, CHEN S, et al. Ferroelectric-enhanced Z-schematic electron transfer in BiVO4-BiFeO3-CuInS2 for efficient photocatalytic pollutant degradation[J]. Applied Catalysis B: Environmental, 2017, 209: 591-599. doi: 10.1016/j.apcatb.2017.03.043
[50] 陈博才, 沈洋, 魏建红, 等.基于g-C3N4的Z-型光催化体系研究进展[J].物理化学学报, 2016(6): 1371-1382. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=wlhxxb201606012 [51] CHEN F, YANG Q, LI X, et al. Hierarchical assembly of graphene-bridged Ag3PO4/Ag/BiVO4 (040) Z-scheme photocatalyst: an efficient, sustainable and heterogeneous catalyst with enhanced visible-light photoactivity towards tetracycline degradation under visible light irradiation[J]. Applied Catalysis B: Environmental, 2017, 200: 330-342. doi: 10.1016/j.apcatb.2016.07.021
[52] IWASE A, NG Y H, ISHIGURO Y, et al. Reduced graphene oxide as a solid-state electron mediator in Z-scheme photocatalytic water splitting under visible light[J]. Journal of the American Chemical Society, 2011, 133(29): 11054-11057. doi: 10.1021/ja203296z
[53] LI X, YAN X, LU X, et al. Photo-assisted selective catalytic reduction of NO by Z-scheme natural clay based photocatalyst: Insight into the effect of graphene coupling[J]. Journal of Catalysis, 2018, 357: 59-68. doi: 10.1016/j.jcat.2017.10.024
[54] LI H, TU W, ZHOU Y, et al. Z-scheme photocatalytic systems for promoting photocatalytic performance: recent progress and future challenges[J]. Advanced Science, 2016, 3(11): 1500389. doi: 10.1002/advs.201500389
[55] TADA H, MITSUI T, KIYONAGA T, et al. All-solid-state Z-scheme in CdS-Au-TiO2 three-component nanojunction system[J]. Nature Materials, 2006, 5(10): 782. doi: 10.1038/nmat1734
[56] XU Q, ZHANG L, YU J, et al. Direct Z-scheme photocatalysts: principles, synthesis, and applications[J]. Materials Today, 2018, 21(10): 1042-1063. doi: 10.1016/j.mattod.2018.04.008
[57] WANG X, LIU G, CHEN Z G, et al. Enhanced photocatalytic hydrogen evolution by prolonging the lifetime of carriers in ZnO/CdS heterostructures[J]. Chemical Communications, 2009(23): 3452-3454. doi: 10.1039/b904668b
[58] YU J, WANG S, LOW J, et al. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air[J]. Physical Chemistry Chemical Physics, 2013, 15(39): 16883-16890. doi: 10.1039/c3cp53131g
[59] ZHOU D, CHEN Z, YANG Q, et al. Facile Construction of g-C3N4 Nanosheets/TiO2 Nanotube Arrays as Z-Scheme Photocatalyst with Enhanced Visible‐Light Performance[J]. ChemCatChem, 2016, 8(19): 3064-3073. doi: 10.1002/cctc.201600828
[60] LIU X, CHEN N, LI Y, et al. A general nonaqueous sol-gel route to g-C3N4-coupling photocatalysts: the case of Z-scheme g-C3N4/TiO2 with enhanced photodegradation toward RhB under visible-light[J]. Scientific Reports, 2016, 6: 39531. doi: 10.1038/srep39531
[61] LIU J, CHENG B, YU J. A new understanding of the photocatalytic mechanism of the direct Z-scheme g-C3N4/TiO2 heterostructure[J]. Physical Chemistry Chemical Physics, 2016, 18(45): 31175-31183. doi: 10.1039/C6CP06147H
[62] BAI S, JIANG J, ZHANG Q, et al. Steering charge kinetics in photocatalysis: intersection of materials syntheses, characterization techniques and theoretical simulations[J]. Chemical Society Reviews, 2015, 44(10): 2893-2939. doi: 10.1039/C5CS00064E
[63] SHAO B, LIU X, LIU Z, et al. A novel double Z-scheme photocatalyst Ag3PO4/Bi2S3/Bi2O3 with enhanced visible-light photocatalytic performance for antibiotic degradation[J]. Chemical Engineering Journal, 2019, 368: 730-745. doi: 10.1016/j.cej.2019.03.013
[64] CONG Y, GE Y, ZHANG T, et al. Fabrication of Z-Scheme Fe2O3-MoS2-Cu2O ternary nanofilm with significantly enhanced photoelectrocatalytic performance[J]. Industrial & Engineering Chemistry Research, 2018, 57(3): 881-890. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=8531e5baebfaaa9be6cabc33f68cf3c4
[65] ZENG D, YANG K, YU C, et al. Phase transformation and microwave hydrothermal guided a novel double Z-scheme ternary vanadate heterojunction with highly efficient photocatalytic performance[J]. Applied Catalysis B: Environmental, 2018, 237: 449-463. doi: 10.1016/j.apcatb.2018.06.010
[66] HAN T, CHEN Y, TIAN G, et al. Hydrogenated TiO2/SrTiO3 porous microspheres with tunable band structure for solar-light photocatalytic H2 and O2 evolution[J]. Science China Materials, 2016, 59(12): 1003-1016. doi: 10.1007/s40843-016-5126-1
[67] NIE N, ZHANG L, FU J, et al. Self-assembled hierarchical direct Z-scheme g-C3N4/ZnO microspheres with enhanced photocatalytic CO2 reduction performance[J]. Applied Surface Science, 2018, 441: 12-22. doi: 10.1016/j.apsusc.2018.01.193
[68] 刘仁月, 吴榛, 白羽, 等.微米球光催化剂在环境净化及能源转化的研究进展[J].有色金属科学与工程, 2016, 7(6): 41-45. http://www.xml-data.org/YSJSYKXGC/html/2016060011.htm [69] 温福宇, 杨金辉, 宗旭, 等.太阳能光催化制氢研究进展[J].化学进展, 2009, 21(11): 2285-2302. http://d.old.wanfangdata.com.cn/Periodical/hndxxbzr201503006 [70] WANG Q, HISATOMI T, JIA Q, et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%[J]. Nature Materials, 2016, 15(6): 611. doi: 10.1038/nmat4589
[71] LIANG Y H, LIAO M W, MISHRA M, et al. Fabrication of Ta3N5/ZnO direct Z-scheme photocatalyst for hydrogen generation[J]. International Journal of Hydrogen Energy, 2019, 44(35): 19162-19167. doi: 10.1016/j.ijhydene.2018.07.117
[72] TIAN L, YANG X, CUI X, et al. Fabrication of dual direct Z-scheme g-C3N4/MoS2/Ag3PO4 photocatalyst and its oxygen evolution performance[J]. Applied Surface Science, 2019, 463: 9-17. doi: 10.1016/j.apsusc.2018.08.209
[73] ZHAO W, LIU J, DENG Z, et al. Facile preparation of Z-scheme CdS-Ag-TiO2 composite for the improved photocatalytic hydrogen generation activity[J]. International Journal of Hydrogen Energy, 2018, 43(39): 18232-18241. doi: 10.1016/j.ijhydene.2018.08.026
[74] WANG S, ZHU B, LIU M, et al. Direct Z-scheme ZnO/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity[J]. Applied Catalysis B: Environmental, 2019, 243: 19-26. doi: 10.1016/j.apcatb.2018.10.019
[75] YUN H J, LEE H, KIM N D, et al. A combination of two visible-light responsive photocatalysts for achieving the Z-scheme in the solid state[J]. ACS nano, 2011, 5(5): 4084-4090. doi: 10.1021/nn2006738
[76] YOU Y, WANG S, XIAO K, et al. Z-scheme g-C3N4/Bi4NbO8Cl heterojunction for enhanced photocatalytic hydrogen production[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(12): 16219-16227. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=148b544c2c70203e5c0c6c3b065781b6
[77] GUO H L, DU H, JIANG Y F, et al. Artificial photosynthetic Z-scheme photocatalyst for hydrogen evolution with high quantum efficiency[J]. The Journal of Physical Chemistry C, 2016, 121(1): 107-114. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=f7ae8ba0b61e755d36808f456979e34f
[78] XU F, ZHANG L, CHENG B, et al. Direct Z-scheme TiO2/NiS core-shell hybrid nanofibers with enhanced photocatalytic H2-production activity[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(9): 12291-12298. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=4a273e5736f7e1fad82f66d94d9f0982
[79] ZOU L, WANG H, WANG X. High efficient photodegradation and photocatalytic hydrogen production of CdS/BiVO4 heterostructure through Z-scheme process[J]. ACS Sustainable Chemistry & Engineering, 2016, 5(1): 303-309. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=a3c6fa884be5bac6f99ce02650eda551
[80] ZHANG L J, LI S, LIU B K, et al. Highly efficient CdS/WO3 photocatalysts: Z-scheme photocatalytic mechanism for their enhanced photocatalytic H2 evolution under visible light[J]. ACS Catalysis, 2014, 4(10): 3724-3729. doi: 10.1021/cs500794j
[81] YE R Q, FANG H B, ZHENG Y Z, et al. Fabrication of CoTiO3/g-C3N4 hybrid photocatalysts with enhanced H2 evolution: Z-scheme photocatalytic mechanism insight[J]. ACS Applied Materials & Interfaces, 2016, 8(22): 13879-13889.
[82] MAEDA K, LU D, DOMEN K. Solar-driven Z-scheme water splitting using modified BaZrO3-BaTaO2N solid solutions as photocatalysts[J]. ACS Catalysis, 2013, 3(5): 1026-1033. doi: 10.1021/cs400156m
[83] IWASE A, YOSHINO S, TAKAYAMA T, et al. Water splitting and CO2 reduction under visible light irradiation using Z-scheme systems consisting of metal sulfides, CoOx-loaded BiVO4, and a reduced graphene oxide electron mediator[J]. Journal of the American Chemical Society, 2016, 138(32): 10260-10264. doi: 10.1021/jacs.6b05304
[84] ARESTA M, DIBENEDETTO A, ANGELINI A. Catalysis for the valorization of exhaust carbon: from CO2 to chemicals, materials, and fuels. Technological use of CO2[J]. Chemical Reviews, 2013, 114(3): 1709-1742. doi: 10.1021/cr4002758
[85] LI X D, SUN Y F, XU J Q, et al. Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers[J]. Nature Energy, 2019, 4(8): 690-699. doi: 10.1038/s41560-019-0431-1
[86] HE Y, ZHANG L, TENG B, et al. New application of Z-scheme Ag3PO4/g-C3N4 composite in converting CO2 to fuel[J]. Environmental Science & Technology, 2014, 49(1): 649-656. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=13944253df7d34cab2c7cb85282ad989
[87] SEKIZAWA K, MAEDA K, DOMEN K, et al. Artificial Z-scheme constructed with a supramolecular metal complex and semiconductor for the photocatalytic reduction of CO2[J]. Journal of the American Chemical Society, 2013, 135(12): 4596-4599. doi: 10.1021/ja311541a
[88] DI T, ZHU B, CHENG B, et al. A direct Z-scheme g-C3N4/SnS2 photocatalyst with superior visible-light CO2 reduction performance[J]. Journal of Catalysis, 2017, 352: 532-541. doi: 10.1016/j.jcat.2017.06.006
[89] WANG J C, YAO H C, FAN Z Y, et al. Indirect Z-scheme BiOI/g-C3N4 photocatalysts with enhanced photoreduction CO2 activity under visible light irradiation[J]. ACS Applied Materials & Interfaces, 2016, 8(6): 3765-3775. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=2524476f8210c96679b03b3dff777482
[90] KUMAR A, PRAJAPATI P K, PAL U, et al. Ternary rGO/InVO4/Fe2O3 Z-scheme heterostructured photocatalyst for CO2 reduction under visible light irradiation[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(7): 8201-8211. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=9ead3174effd54f08b346223e854f1ad
[91] WANG J C, ZHANG L, FANG W X, et al. Enhanced photoreduction CO2 activity over direct Z-scheme α-Fe2O3/Cu2O heterostructures under visible light irradiation[J]. ACS Applied Materials & Interfaces, 2015, 7(16): 8631-8639. https://www.researchgate.net/publication/274643452_Enhanced_Photoreduction_CO2_Activity_over_Direct_Z-Scheme_-Fe2O3Cu2O_Heterostructures_Under_Visible_Light_Irradiation
[92] BHOSALE R, JAIN S, VINOD C P, et al. Direct Z-Scheme g-C3N4/FeWO4 nanocomposite for enhanced and selective photocatalytic CO2 reduction under visible light[J]. ACS Applied Materials & Interfaces, 2019, 11(6): 6174-6183. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=5fcd5e5b049798e23383d6024f62a00a
[93] JIN J, YU J, GUO D, et al. A Hierarchical Z-scheme CdS-WO3 photocatalyst with enhanced CO2 reduction activity[J]. Small, 2015, 11(39): 5262-5271. doi: 10.1002/smll.201500926
[94] WU F, LI X, LIU W, et al. Highly enhanced photocatalytic degradation of methylene blue over the indirect all-solid-state Z-scheme g-C3N4-RGO-TiO2 nanoheterojunctions[J]. Applied Surface Science, 2017, 405: 60-70. doi: 10.1016/j.apsusc.2017.01.285
[95] HE Y, ZHANG L, FAN M, et al. Z-scheme SnO2-x/g-C3N4composite as an efficient photocatalyst for dye degradation and photocatalytic CO2 reduction[J]. Solar Energy Materials and Solar Cells, 2015, 137: 175-184. doi: 10.1016/j.solmat.2015.01.037
[96] JO W K, SELVAM N C S. Z-scheme CdS/g-C3N4 composites with RGO as an electron mediator for efficient photocatalytic H2production and pollutant degradation[J]. Chemical Engineering Journal, 2017, 317: 913-924. doi: 10.1016/j.cej.2017.02.129
[97] XIE Z, FENG Y, WANG F, et al. Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline[J]. Applied Catalysis B: Environmental, 2018, 229: 96-104. doi: 10.1016/j.apcatb.2018.02.011
[98] LU D, WANG H, ZHAO X, et al. Highly efficient visible-light-induced photoactivity of Z-scheme g-C3N4/Ag/MoS2 ternary photocatalysts for organic pollutant degradation and production of hydrogen[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(2): 1436-1445. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=a087085deb188800b8cf23a04a8e97ea
[99] TANG H, FU Y, CHANG S, et al. Construction of Ag3PO4/Ag2MoO4 Z-scheme heterogeneous photocatalyst for the remediation of organic pollutants[J]. Chinese Journal of Catalysis, 2017, 38(2): 337-347. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201702018
[100] YANG Y, GUO W, GUO Y, et al. Fabrication of Z-scheme plasmonic photocatalyst Ag@AgBr/g-C3N4 with enhanced visible-light photocatalytic activity[J]. Journal of Hazardous Materials, 2014, 271: 150-159. doi: 10.1016/j.jhazmat.2014.02.023
[101] HE R, CHENG K, WEI Z, et al. Room-temperature in situ fabrication and enhanced photocatalytic activity of direct Z-scheme BiOI/g-C3N4 photocatalyst[J]. Applied Surface Science, 2019, 465: 964-972. doi: 10.1016/j.apsusc.2018.09.217
[102] HE R, ZHOU J, FU H, et al. Room-temperature in situ fabrication of Bi2O3/g-C3N4 direct Z-scheme photocatalyst with enhanced photocatalytic activity[J]. Applied Surface Science, 2018, 430: 273-282. doi: 10.1016/j.apsusc.2017.07.191
[103] WANG F, LI W, GU S, et al. Facile fabrication of direct Z-scheme MoS2/Bi2WO6 heterojunction photocatalyst with superior photocatalytic performance under visible light irradiation[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2017, 335: 140-148. doi: 10.1016/j.jphotochem.2016.11.026
[104] LIN H, CA J, LUO B, et al. Synthesis of novel Z-scheme AgI/Ag/AgBr composite with enhanced visible light photocatalytic activity[J]. Catalysis Communications, 2012, 21: 91-95. doi: 10.1016/j.catcom.2012.02.008
[105] LI W, CHEN J, GUO R, et al. Facile fabrication of a direct Z-scheme MoO3/Ag2CrO4 composite photocatalyst with improved visible light photocatalytic performance[J]. Journal of Materials Science: Materials in Electronics, 2017, 28(21): 15967-15979. doi: 10.1007/s10854-017-7495-0
[106] LIU H, DU C, BAI H, et al. Fabrication of plate-on-plate Z-scheme SnS2/Bi2MoO6 heterojunction photocatalysts with enhanced photocatalytic activity[J]. Journal of Materials Science, 2018, 53(15): 10743-10757. doi: 10.1007/s10853-018-2296-2
[107] UTKA A, VANAGS M, JOOST U, et al. Aqueous synthesis of Z-scheme photocatalyst powders and thin-film photoanodes from earth abundant elements[J]. Journal of Environmental Chemical Engineering, 2018, 6(2): 2606-2615. doi: 10.1016/j.jece.2018.04.003
[108] WU X F, LI H, PAN J C, et al. Designing visible-light-driven direct Z-scheme Ag2WO4/WS2 heterojunction to enhance photocatalytic activity[J]. Journal of Materials Science: Materials in Electronics, 2018, 29(17): 14874-14882. doi: 10.1007/s10854-018-9625-8
[109] QIAO Q, HUANG W Q, LI Y Y, et al. In-situ construction of 2D direct Z-scheme g-C3N4/g-C3N4 homojunction with high photocatalytic activity[J]. Journal of Materials Science, 2018, 53(23): 15882-15894. doi: 10.1007/s10853-018-2762-x
[110] DING J, DAI Z, QIN F, et al. Z-scheme BiO1-xBr/Bi2O2CO3 photocatalyst with rich oxygen vacancy as electron mediator for highly efficient degradation of antibiotics[J]. Applied Catalysis B: Environmental, 2017, 205: 281-291. doi: 10.1016/j.apcatb.2016.12.018
[111] HEZAM A, NAMRATHA K, PONNAMMA D, et al. Direct Z-scheme Cs2O-Bi2O3-ZnO heterostructures as efficient sunlight-driven photocatalysts[J]. ACS Omega, 2018, 3(9): 12260-12269. doi: 10.1021/acsomega.8b01449
[112] HUANG Z, ZENG X, LI K, et al. Z-scheme NiTiO3/g-C3N4 heterojunctions with enhanced photoelectrochemical and photocatalytic performances under visible LED light irradiation[J]. ACS Applied Materials & Interfaces, 2017, 9(47): 41120-41125. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3a828be640c4f6b56ce4fe5c460b7053
[113] LI Q, GUAN Z, WU D, et al. Z-scheme BiOCl-Au-CdS heterostructure with enhanced sunlight-driven photocatalytic activity in degrading water dyes and antibiotics[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(8): 6958-6968. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=b80fb822b8a22c5aa759d0dd5efb2a4e
[114] LI C, YU S, CHE H, et al. Fabrication of Z-scheme heterojunction by anchoring mesoporous γ-Fe2O3 nanospheres on g-C3N4 for degrading tetracycline hydrochloride in water[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(12): 16437-16447.
[115] 熊威, 葛建华, 陈羽冲, 等. g-C3N4光催化还原Cr(Ⅵ)研究进展[J].广州化工, 2018, 46(1): 12-14. doi: 10.3969/j.issn.1001-9677.2018.01.006 [116] WIEDERHOLD J G. Metal stable isotope signatures as tracers in environmental geochemistry[J]. Environmental Science & Technology, 2015, 49(5): 2606-2624. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=6f4f747a94434900d6dc26eea3fce753
[117] ZHOU Y, CHEN G, YU Y, et al. A new oxynitride-based solid state Z-scheme photocatalytic system for efficient Cr(VI) reduction and water oxidation[J]. Applied Catalysis B: Environmental, 2016, 183: 176-184. doi: 10.1016/j.apcatb.2015.10.040
[118] CHEN A, BIAN Z, XU J, et al. Simultaneous removal of Cr(VI) and phenol contaminants using Z-scheme bismuth oxyiodide/reduced graphene oxide/bismuth sulfide system under visible-light irradiation[J]. Chemosphere, 2017, 188: 659-666. doi: 10.1016/j.chemosphere.2017.09.002
[119] CHEN F, YANG Q, WANG Y, et al. Efficient construction of bismuth vanadate-based Z-scheme photocatalyst for simultaneous Cr (VI) reduction and ciprofloxacin oxidation under visible light: Kinetics, degradation pathways and mechanism[J]. Chemical Engineering Journal, 2018, 348: 157-170. doi: 10.1016/j.cej.2018.04.170
[120] YU C, CHEN F, ZENG D, et al. A facile phase transformation strategy for fabrication of novel Z-scheme ternary heterojunctions with efficient photocatalytic properties[J]. Nanoscale, 2019, 11(16): 7720-7733. doi: 10.1039/C9NR00709A
-
期刊类型引用(0)
其他类型引用(1)




 下载:
下载: