Influence of CaO content in slag CaF2-Al2O3-CaO-Ce2O3 on the behavior of Ce2O3
-
摘要: 在Fe-Cr-Al合金中添加微量的稀土元素可显著改善合金的使用性能, 文中以电渣重熔生产Fe-Cr-Al合金所用稀土渣为研究对象, 根据离子-分子共存理论(IMCT)建立了1 823 K时CaF2-Al2O3-CaO-Ce2O3四元渣系热力学质量作用浓度模型.结果表明:当渣系中CaO和Al2O3的质量百分数之比维持在1:1, 即wCaO /wAl2O3=1时, 随着Ce2O3含量增加, 渣中铈铝酸盐Ce2O3·Al2O3质量作用浓度(活度)显著增加, 但炉渣物相种类没有变化.渣系中Ce2O3含量分别在10 %, 20 %, 30 %, 40 %时, wCaO /wAl2O3值对组元活度的影响各不相同但有共同特征, 表现在处于约1.0~1.8时, CaO活度增加最迅速, Ce2O3活度增加, Ce2O3·Al2O3活度下降, 说明渣中CaO含量增加促进了Ce2O3·Al2O3分解而导致Ce2O3活度增加, 选取渣系进行熔融和X射线衍射实验, 用jade 5.0软件分析物相, 实验结果与计算一致.Abstract: The performance of Fe-Cr-Al alloy can be improved significantly when it contains a small amount of rare earth. In this paper, after studying the slags used for producing Fe-Cr-Al alloy with the method of ESR, we then establish the thermodynamic model of mass action concentration of CaF2-Al2O3-CaO-Ce2O3 at 1873 K based on ion and molecule coexistence theory (IMCT). The result demonstrates that the value of Ce2O3·Al2O3 activity increases when keeping and increasing Ce2O3 content in slags, but the phase composition in slags does not change; when the concentration of Ce2O3 is 10 %, 20 %, 30 %, 40 % respectively, the value of differs and poses different impacts on the activity of compositions, but when the value is between 1.0 and 1.8, the activity of CaO increases fastest and that of Ce2O3·Al2O3 decreases, which demonstrates the decomposition role of CaO in slags is remarkable. We select slags to conduct melting and XRD experiment on the samples and then use jade5.0 software to analyze the result, which proves to be consistent with the theory.
-
Keywords:
- coexistence theory /
- mass action concentrations /
- diffraction /
- rare earth
-
Fe-Cr-Al铁素体不锈钢合金, 具有电阻率系数大、电阻温度系数小、良好的耐热性和抗高温氧化性能及生产成本低等优点, 被大量用于电热、电信、核电领域等, 在合金中加入稀土元素可以大幅度提高其高温使用性能[1-3].用电渣重熔法生产Fe-Cr-Al合金常用CaF2-Al2O3-CaO三元渣系[4-6].为提高合金中稀土元素含量, 生产中向渣中加入富含稀土Ce的氧化物而形成CaF2-Al2O3-CaO-Ce2O3四元渣系, 有关CaF2-Al2O3-CaO-Ce2O3四元渣系的研究目前国内外报道极少, 随着稀土元素更多的应用于钢铁和有色金属材料中, 对含稀土的渣系研究已十分必要, 但稀土元素活性强在冶炼过程烧损严重, 稀土氧化物热力学性质较难测定, 热力学软件有关稀土化合物的热力学数据目前严重缺失.
前人[7-8]曾做过Al2O3-CaO-Ce2O3三组元的热力学研究, 但非电渣重熔渣系.自炉渣结构离子-分子共存理论(IMCT)提出以来, 在不同渣系都有运用, 尤其在冶金领域取得了很大进步, DUAN[9]根据此理论将质量作用浓度代替传统活度方法在学术界阐明了渣钢中MnO的分配规律, 史成斌等[10]利用此理论定量计算出了自由态CaO和自由态MgO各自对炉渣脱硫的贡献, 段生朝等[11]根据此理论计算出Bi-Pb二元合金中金属间化合物BiPb的标准生成吉布斯自由能表达式.目前该理论在CaF2-Al2O3-CaO-Ce2O3四元渣系中尚无应用, 文中基于离子-分子共存理论研究冶炼稀土Fe-Cr-Al高温合金所用CaF2-Al2O3-CaO-Ce2O3四元渣, 建立热力学模型, 研究渣中CaO含量的变化对组元Ce2O3行为的影响.
1 热力学模型
1.1 热力学模型的建立
查阅Ce2O3-Al2O3, Al2O3-CaO, Al2O3-CaO-Ce2O3, CaF2-Al2O3-CaO等相图[8, 12-15], 基于离子-分子共存理论建立CaF2-Al2O3-CaO-Ce2O3四元渣系中结构单元和离子对质量作用浓度热力学模型, 需做如下假设:①在熔化温度下, 液态炉渣中存在3类结构单元:简单离子Ca2+、Ce3+、O2-、F-; 简单分子Al2O3; 复杂分子CaO·Al2O3、CaO·2Al2O3、CaO·6Al2O3、3CaO·Al2O3、12CaO·7Al2O3、CaF2·3Al2O3·3CaO[15]、CaF2·7Al2O3·11CaO[15]、Ce2O3·Al2O3、Ce2O3·11Al2O3共9种, 其中Ce元素以2种化合物形式存在Ce2O3·Al2O3和Ce2O3·11Al2O3, 熔渣中阳离子和阴离子各占一个结构单元, 以离子对的形式参与化学反应. ②离子对与简单分子之间形成复杂分子的过程是一个动态平衡过程.如铝酸钙盐的形成过程为(Ca2++O2-)+Al2O3=CaO·Al2O3, 将Ca2+和O2-加括号的原因是因为CaO在固态下以离子晶格存在(类似NaCl结构), 由固态到液态离子Ca2+和O2-始终保持独立而不形成分子. ③渣系中的结构单元在所研究的浓度范围内具有连续性. ④渣系中发生的化学反应均服从质量作用定律, 每种结构单元的质量作用浓度就是实测的活度.其中离子对CaO、Ce2O3、CaF2的质量作用浓度用N1、N2、N3表示, 简单分子Al2O3用N4表示, 复杂分子CaO·Al2O3、CaO·2Al2O3 …Ce2O3·11Al2O3用N5、N6、…、N13表示.
四元渣系CaF2-Al2O3-CaO-Ce2O3中结构单元及其摩尔数和作用浓度表达式如表 1所列, 可能形成复杂分子的化学反应表达式见表 2.
表 1 CaF2-Al2O3-CaO-Ce2O3四元渣系中结构单元及摩尔分数和作用浓度表达式Table 1. Expression of structural units, mole numbers, and mass action concentrations of CaF2-Al2O3-CaO-Ce2O3slag based on IMCT 表 2 可能形成复杂分子的化学表达式Table 2. Chemical reaction formulas of possibly formed complex molecules
表 2 可能形成复杂分子的化学表达式Table 2. Chemical reaction formulas of possibly formed complex molecules
1.2 模型方程及解
根据离子-分子共存理论的上述4点假设, 设初始四元渣系各自摩尔质量分别为

(1) 
(2) 
(3) 
(4) 
(5) 上述5个方程中独立变量有N1、N2、N3、N4、∑Ni共5个, 用MATLAB软件中Fslove功能可得方程唯一解.需要指出的是CaO、Ce2O3、CaF2作为独立组分没有质量作用浓度, 以CaO为例, CaO的质量作用浓度是Ca2+和O2-两种结构单元质量作用浓度之和, 用来表征自由CaO的反应能力[10].
1.3 渣系组元的选择
电渣冶金所用渣系需要综合考虑炉渣的熔化温度, 软化区间, 流动性, 导电率, 高温热稳定性和吸收夹杂物能力等, 研究表明[18-22], 使用多组元渣系所得到的钢中含有的Al2O3夹杂物的尺寸比传统的二元系更小, 降低Al2O3活度并提高精炼渣的流动性有利于炉渣对钢中Al2O3夹杂物的吸收, Ce2O3含量的增加对四元渣系物理化学性能改变较小, 在多元渣系中Ce2O3的含量有较宽的适用范围[7], 由相图[12-13]可知Al2O3-CaO-Ce2O3三元渣系在温度为1 773 K时, Ce2O3最大溶解度为45 %, 且液相区较窄, 此时Al2O3与CaO质量分数之比约1:1, 综合以上因素选择渣系进行计算.所选渣系分为2个变量, 其一为增加渣中Ce2O3质量百分数并保持Al2O3和CaO含量不变, 且wCaO/wAl2O3=1, 另一个为保持渣中Ce2O3不变(10 %, 20 %, 30 %, 40 %), 增加CaO与Al2O3的比值.
2 计算结果分析
2.1 wCaO/wAl2O3=1时, 渣中组元活度变化与渣中Ce2O3含量关系
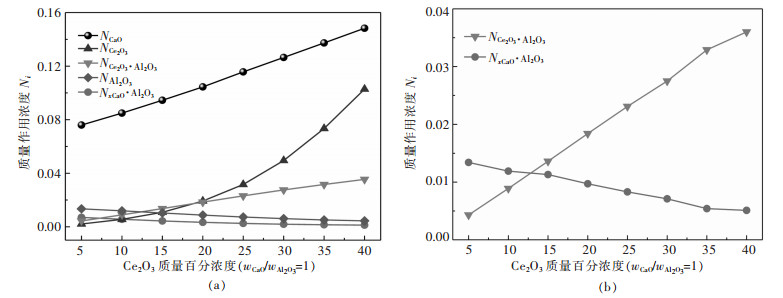
由于组元CaF2质量作用浓度最大(约为0.7)且变化较小, 故不和其他组元对比, 计算结果如图 1, 可以看出当wCaO/wAl2O3=1时, 随着熔渣中Ce2O3质量百分数的增加, CaO的质量作用浓度(活度)值最大且稳定增加, Ce2O3质量作用浓度变化可以分为2个阶段, 当时wCe2O3≤20 %, NCe2O3≤NCe2O3·Al2O3, 当wAl2O3>20 %时, NCe2O3>NCe2O3·Al2O3且此时NCe2O3迅速增加, Ce2O3·11Al2O3的质量作用浓度极小(NCe2O3·11Al2O3=2.3×10-22), 说明Al2O3始终以Ce2O3·Al2O3化合物的形式持续消耗着Ce2O3, 溶液几乎不含化合物Ce2O3·11Al2O3, 其中当wCe2O3≤20 %时特征最明显, NAl2O3逐渐降低且接近零.渣中钙铝酸盐的复杂分子化合物如CaO·Al2O3、CaO·2Al2O3、CaO·6Al2O3、3CaO·Al2O3等由于它们质量作用浓度小, 将其加和后用NxCaO·Al2O3表示, 渣中存在两类分子化合物一类为铈铝酸盐Ce2O3·Al2O3, 另一类为钙铝酸盐xCaO·Al2O3, 随着渣中Ce2O3质量分数的增加, NCe2O3·Al2O3迅速增加, 但NxCaO·Al2O3逐渐降低, 如图 1(b).
2.2 wCaO/wAl2O3值对不同Ce2O3含量的炉渣中组元活度的影响
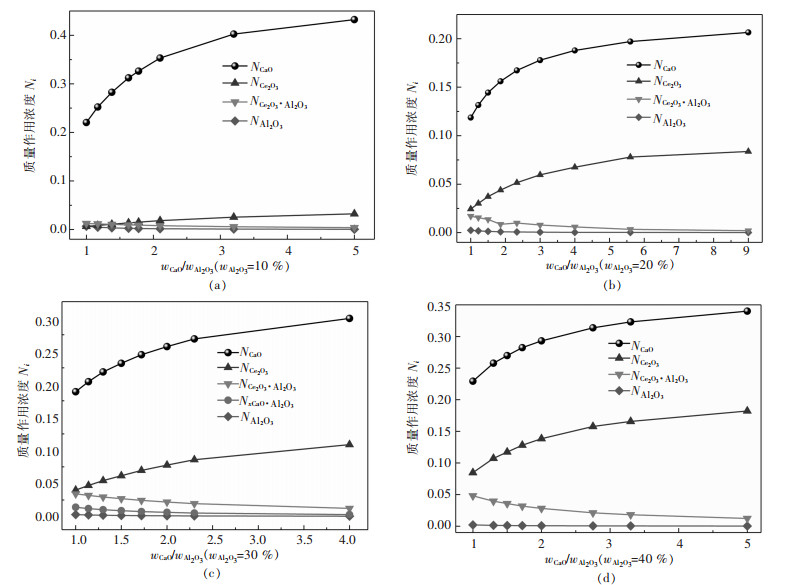
wCaO /wAl2O3值对炉渣中组元活度影响的计算结果如图 2, 从图 2(a)可以看出, 当wCe2O3=10 %时, 随着wCaO /wAl2O3值的增加NCe2O3缓慢增加, NCe2O3·Al2O3的值与NCe2O3起始数值比较接近, 但随后逐渐降低, 说明铈元素由铈铝酸盐分子转向离子态, NAl2O3变化较小且接近零, NCaO值在wCaO /wAl2O3=1.0~1.8时迅速增加, 随后缓慢增加, 图 2(b)中wCe2O3=20 %, 可以看出随着wCaO/wAl2O3值的增加, NCe2O3快速增加, NCe2O3·Al2O3的值与NCe2O3起始数值接近区域只在起点, 说明更多铈元素处于离子态, NCe2O3·Al2O3对NCe2O3的影响已不显著, NAl2O3变化较小, NCaO在wCaO/wAl2O3=1.0~2.0时迅速增加, 此时CaO活性较大, 随后增加变缓; 图 2(c)说明, 当wCe2O3=30 %时随着wCaO/wAl2O3值增加, NCe2O3快速增加, 但NCe2O3·Al2O3明显减少, 说明铈铝酸盐Ce2O3·Al2O3分子被分解, 导致NCe2O3·Al2O3增加, 在NCe2O3·Al2O3=1.0~2.0时迅速增加; 图 2(d)表明, 当wCe2O3=40 %时随着wCaO/wAl2O3值增加, NCe2O3快速增加, NAl2O3, NCe2O3·Al2O3与值的变化与图 2(b)、图 2(c)相似, NCaO在wCaO /wAl2O3=1.0~1.8时迅速增加, 说明此时CaO分解铈铝酸盐分子的能力最强.由此可知, 当渣中Ce2O3含量恒定时, 增加CaO含量可以分解铈铝酸盐Ce2O3·Al2O3.
wCaO/wAl2O3值对渣系物理化学性能有重要影响, CaO活度增加后自由态离子Ca2+和O2-增加, 其中Ca2+的离子半径最小(r(Ca2+)=0.99Ǻ, r(Ce2+)=1.03Ǻ, r(O2)=1.44Ǻ), 扩散能力强.王韬等[23]研究CaO稳定氧化锆中氧扩散行为, 表明O2-的扩散系数在富钙区域的扩散系数比低钙区域扩散系数高. QI等[24]发现, Ce2O3的增加可以促进炉渣中复杂结构单元的解离, wCaO/wAl2O3值在0.6~1.4时炉渣表面张力增加. XU等[25]研究表明, wCaO/wAl2O3值的影响着渣系物相变化, 改变了体系活化能并对熔化温度产生较大影响, 其值在1.1~1.3变化时渣系的活化能变化最剧烈. wCaO/wAl2O3=1.5时, 含Ce2O3四元渣具有较低的熔化温度[7].文中计算显示wCaO/wAl2O3值在约1.0~1.8时, CaO和Ce2O3质量作用浓度(活度)迅速上升, 而Ce2O3·Al2O3下降, 说明此时CaO的分解Ce2O3·Al2O3的作用最强.
3 结果和讨论
3.1 热力学模型和渣中物相
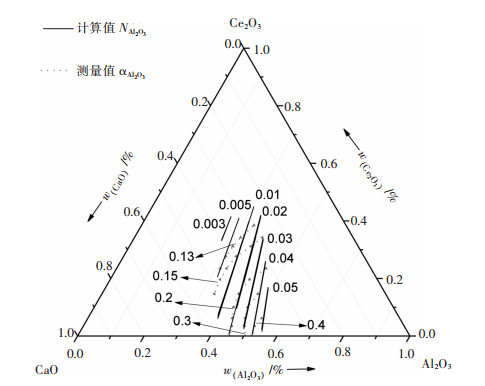
目前的检测手段很难测出熔渣在1 823 K时, 渣中某一组元活度的准确值, 含Ce2O3的液态炉渣中关于组元活度测量的报道极少, SHIGERU[8, 12]实测了Al2O3-CaO-Ce2O3三元渣系在温度为1 773 K时, 改变组元不同配比时Al2O3的活度值, 将热力学模型计算出的组元Al2O3质量作用浓度值与实测活度值对比, 结果如图 3所示, 可知计算值与实测值相差约10倍, 但两者变化趋势一致, 数据差别较大的原因是因为两者所取标准态不同, 活度测量值选取组元质量分数1 %为标准态, 而质量作用浓度计算值是以组元纯物质为标准态.
由计算可知, wCaO/wAl2O3=1时, 渣中存在两类分子化合物, 其中一类为铈铝酸盐Ce2O3·Al2O3, 另一类为钙铝酸盐xCaO·Al2O3, 随着渣中Ce2O3质量百分数的增加, Ce2O3·Al2O3活度迅速增加, xCaO·Al2O3逐渐降低, 渣中Ce2O3含量恒定时, 增加CaO可以分解铈铝酸盐, X射线衍射方法可以测出渣中相的组成和该相含量变化情况[26-27].
3.2 实验过程
渣料由分析纯(纯度>99.9 %)CaF2、Al2O3、CaO化学试剂配制(粒度为1~5 μm), 其中Ce2O3由铝粉还原CeO2所得[7], 按比例配好后装入石墨坩埚中, 用博莱曼特blmt-12型Si-Mo管式炉(φ90 mm×1 000 mm)为反应加热装置, 炉内反应管恒温带长度为20 cm, 温度采用PID自动控制, 控制精度为±1 ℃, 反应温度由坩埚底部的测温热电偶获得, 实验时(温度升至600 ℃时)由炉管底部通入高纯氩气进行气氛保护, 流量为(180±20) mL/min.制取试样时每次配制50 g炉渣, 反应温度为1 550 ℃(1 823 K), 升温程序设置为600 ℃之前升温速率为6 ℃/min, 600~1 550 ℃时升温速率为4 ℃/min, 升温至1 550 ℃时将石墨坩埚放入管式炉中, 保温时间2 h, 反应结束后将坩埚中取出放入水中淬火[13], 样品用磨盘机磨成粉体, 进行衍射实验, 仪器为日本理学Ultima-Ⅳ3KW X射线衍射仪, Cu靶材, 波长1.54 Ǻ, 工作电压40 kV, 工作电流40 mA, 探测器为Ni滤波片+D/teX-Ultra高速探测器, 扫描速度为16°/min.其中所配炉渣的成分如表 3所列, 分别编号为S1、S2、S3、S4, XRD实验结果用jade5.0分析物相.
表 3 实验渣系成分/(质量百分数, %)Table 3. Component list of experimental slags /(mass percent, %)
3.3 实验结果与讨论
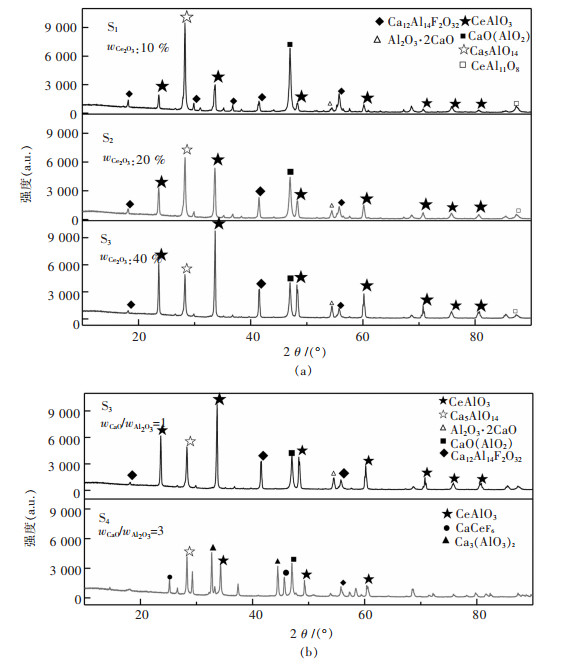
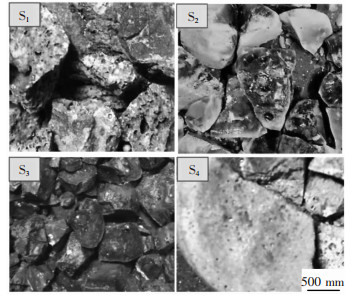
渣中wCaO/wAl2O3=1:1时, 物相XRD检测结果如图 4(a)所示, 图 4(b)为wCaO/wAl2O3值分别为1和3时渣中物相对比图, 实际熔渣照片如图 5, 结合生产实际讨论CaF2-Al2O3-CaO-Ce2O3四元渣在wCaO/wAl2O3=1:1和wCaO/wAl2O3=3:1时渣中物相变化情况.
当wCaO/wAl2O3=1:1时, Ce2O3的含量从10 %增加至40 %, S1→S2→S3, 渣中物相Ce2O3·Al2O3(2CeAlO3)显著增加, 其特征峰越来越高, 越来越强, 但物相种类没有变化.说明渣中随着渣中组元Ce2O3含量的增加, 形成更多渣相Ce2O3·Al2O3(2CeAlO3), 渣中Ce2O3含量增加, 意味着消耗更多的稀土资源和更高冶炼成本, 观察实物可以发现含铈炉渣颜色为绿色, 有分层现象, Ce2O3质量分数为20 %时(S2)分层现象最明显, 白绿相间, 文献[8]报道绿色区域为富铈区域而白色区几乎不含铈, 分层原因尚不清楚.
当wCaO/wAl2O3=3:1时, 渣中铈铝酸盐相明显减少, 含铈氟化物和钙铝酸盐增加.表明CaO可将渣中Ce2O3·Al2O3(2CeAlO3)分子破坏, 其特征峰强度显著降低如图 4(b).该实验与KITANO所得实验结果相似, KITANO研究三元渣系Al2O3-CaO-Ce2O3组元活度并分析了渣中化合物的晶体结构, 进行了wCaO/wAl2O3为1和1/3时的熔融实验(1 823 K)和XRD检测, 结果为渣中含铈主要复合化合物为CaO·Ce2O3·Al2O3(CACe)和CaO·Ce2O3·3Al2O3(CA3Ce).复合化合物存在原因可能在于该三元渣系中不含CaF2, F-结构简单扩散能力强, CaF2和CaO共同作用分解稀土氧化物的能力强于单一的CaO[28-29], 渣中较高的Al2O3含量有利于形成化合物CaO·Ce2O3·xAl2O3.
在实际生产中, 铁铬铝合金含有适量Ce元素(约0.04 %), 可以同时增强合金的室温塑性和屈服强度, 明显提高合金高温抗蠕变性能, 增加渣中CaO可以提高铁铬铝合金中稀土钇镧铈等元素的含量[30-31].
4 结论
1) 根据离子-分子共存理论(IMCT)建立了CaF2-Al2O3-CaO-Ce2O3渣系在1 823 K时质量作用浓度模型, 计算表明当渣中Ce2O3含量分别为10 %, 20 %, 30 %, 40 %时, wCaO/wAl2O3值在约1.0~1.8, CaO分解铈铝酸盐的作用最显著, 表现在CaO质量作用浓度增加最迅速, Ce2O3·Al2O3被分解其质量作用浓度逐渐降低, Ce2O3质量作用浓度快速提高.
2) 炉渣衍射实验表明, 当wCaO/wAl2O3=1:1时, CaF2-Al2O3-CaO-Ce2O3渣系中Ce2O3·Al2O3(2CeAlO3)相随着Ce2O3的含量而显著增加, 物相种类没有变化.增加渣中CaO含量是破坏渣中Ce2O3·Al2O3(2CeAlO3)的有效手段(1 823 K).
-
表 1 CaF2-Al2O3-CaO-Ce2O3四元渣系中结构单元及摩尔分数和作用浓度表达式
Table 1 Expression of structural units, mole numbers, and mass action concentrations of CaF2-Al2O3-CaO-Ce2O3slag based on IMCT

表 2 可能形成复杂分子的化学表达式
Table 2 Chemical reaction formulas of possibly formed complex molecules

表 3 实验渣系成分/(质量百分数, %)
Table 3 Component list of experimental slags /(mass percent, %)

-
[1] DRYEPONDT S, TURAN J, LEONARD D, et al. Long-term oxidation testing and lifetime modeling of cast and ODS FeCrAl Alloys[J]. Oxidation of Metals, 2017, 87(1/2):215-248. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cf955bad11895721bdf91fc4fd315750
[2] REBAK R B, GUPTA V K, LARSEN M. Oxidation Characteristics of two FeCrAl alloys in air and steam from 800℃ to 1300℃[J].Journal of The Minerals, Metals & Materials Society, 2018, 70(8):1484-1492.
[3] CHECMANOWSKI J, PELCZARSKA A J, SZCYGIEL I, et al.Influence of ceria and yttria on the protective properties of SiO2-Al2O3 coatings deposited by sol-gel method on FeCrAl alloy[J].Journal of Thermal Analysis & Calorimetry, 2016, 126(2):1-10. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=45a73cd3848cac06d959f523a9d748d5
[4] 赵鸿燕, 冯浩, 李花兵, 等.渣系对Ar气保护电渣重熔Ni-Cr-Co基高温合金质量的影响[J].材料与冶金学报, 2013, 12(2):119-123. http://d.old.wanfangdata.com.cn/Periodical/clyyjxb201302008 [5] BUSCH J D, DEBARBADILLO J J, KRANE M J M.Flux entrapment and titanium nitride defects in electroslag remelting of INCOLOY alloys 800 and 825[J]. Metallurgical & Materials Transactions A Physical Metallurgy & Materials Science, 2013, 44(12):5295-5303. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=67a7db51e4fd6c02bc17d5a7e977d5f0
[6] 姜周华, 董艳伍, 耿鑫, 等.电渣冶金学[M].北京:科学出版社, 2015. [7] 龙鹄, 成国光, 吴彬, 等.含Ce2O3炼钢精炼渣熔化及流动特性的研究[J].中国稀土学报, 2010, 28(6):721-727. http://d.old.wanfangdata.com.cn/Periodical/zgxtxb201006014 [8] 吴铖川, 成国光, 龙鹄.含Ce2O3渣系作用浓度的计算模型[J].中国有色金属学报, 2013(10):2999-3005. http://d.old.wanfangdata.com.cn/Periodical/zgysjsxb201310035 [9] DUAN S, LI C, GUO X, et al. A thermodynamic model for calculating manganese distribution ratio betweenCaO-SiO2-MgO-FeO-MnO-Al2O3-TiO2-CaF2 ironmaking slags and carbon saturated hot metal based on the IMCT[J]. Ironmaking & Steelmaking, 2018, 45(7):655-664.
[10] 史成斌, 郭汉杰, 丁汝才, 等.CaO-SiO2-MgO-Al2O3渣系与碳饱和铁液间硫分配比的热力学模型[J].过程工程学报, 2010, 10(增刊1):158-164. http://d.old.wanfangdata.com.cn/Periodical/hgyj2010z1033 [11] 段生朝, 郭汉杰, 郭靖, 等.基于原子-分子理论的二元合金熔体热力学计算[J].有色金属科学与工程, 2017, 8(3):7-15. http://ysjskx.paperopen.com/oa/DArticle.aspx?type=view&id=201611010 [12] SHIGERU U A, KAZUKI M T, NOBUO S N. Activity of AlO1.5 for the CaO-AlO1.5-CeO1.5 system at 1773K[J].ISIJ International, 1998, 38(12):1292-1296. doi: 10.2355/isijinternational.38.1292
[13] KITANO R, ISHII M, UO M, et al. Thermodynamic properties of the CaO-AlO1.5-CeO1.5 system[J]. ISIJ International, 2016, 56(11):1893-1901. doi: 10.2355/isijinternational.ISIJINT-2016-201
[14] 李星, 耿鑫, 姜周华, 等.电渣重熔高温合金渣系对冶金质量的影响[J].钢铁, 2015, 50(9):41-46. http://d.old.wanfangdata.com.cn/Periodical/gt201509007 [15] CHATTERJEE A K, ZHMOIDING I. The phase equilibrium diagram of the system CaO-Al2O3-CaF2[J]. Journal of Materials Science, 1972, 7(1):93-97. doi: 10.1007/BF00549555
[16] YANG X M, SHI C B, ZHANG M, et al. A Thermodynamic model for prediction of Iron oxide activity in some FeO-containing Slag Systems, Steel Research International, 2012, 83(3);244-258.
[17] JIANG Z H, HOU D, DONG Y W, et al. Effect of slag on titanium, silicon, and aluminum contents in superalloy During electroslag remelting[J]. Metallurgical and Materials Transactions B, 2016, 47(2):1465-1474. doi: 10.1007/s11663-015-0530-8
[18] 王光迪, 张福臣.渣系对电渣钢夹杂物的影响[J].特殊钢, 1984, (2):21-26. http://cdmd.cnki.com.cn/Article/CDMD-10145-1015702629.htm [19] 杨文晟, 吴建中, 郭汉杰, 等. DH36钢中碳氮化物析出的热力学分析[J].有色金属科学与工程, 2017, 8(1):29-34. http://ysjskx.paperopen.com/oa/DArticle.aspx?type=view&id=201404032 [20] 刘伟, 赖朝彬, 冯小明, 等.无氟预熔型精炼渣的设计与应用[J].有色金属科学与工程, 2012, 3(5):45-49. http://ysjskx.paperopen.com/oa/DArticle.aspx?type=view&id=201209003 [21] 佟志芳, 姜喜远, 陈涛, 等.炉渣组分对CaO-Al2O3-SiO2-TiO2-MgO-Na2O渣系与铁液间硫分配比的影响[J].有色金属科学与工程, 2016, 7(1):5-10. http://ysjskx.paperopen.com/oa/DArticle.aspx?type=view&id=201509006 [22] 于梦曦, 郭汉杰, 郭靖, 等.电渣冶金熔炼Fe-Cr-Al高温合金抑制镧、铈稀土氧化的研究[J].材料与冶金学报, 2016, 15(4):247-253. http://d.old.wanfangdata.com.cn/Periodical/clyyjxb201604003 [23] 王韬, 韩露, 王森, 等. CaO稳定氧化锆中氧扩散行为的分子动力学模拟[J].陶瓷学报, 2018.39(5):592-595. http://d.old.wanfangdata.com.cn/Periodical/tcxb201805013 [24] QI J, LIU C, JIANG M. Evaluation of surface tension of Ce-bearing slag based on calcium aluminate system[J]. Ironmaking & Steelmaking, 2018, 45(44):342-349. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1080/03019233.2016.1265804
[25] XU J F, ZHANG J Y, JIE C, et al.Experimental measurements and modelling of viscosity in CaO-Al2O3-MgO slag system[J].Ironmaking & Steelmaking, 2013, 38(5):329-337.
[26] 辛艳青, 杨田林, 宋淑梅, 等.基于X射线衍射物相定量分析外标法的研究[J].科技创新与应用, 2013, 34:2-3. http://d.old.wanfangdata.com.cn/Periodical/qgsj201334002 [27] 刘洋, 束奇峰.MgO和MnO对CaO-SiO2-Fe2O3-P2O5渣的物相影响[J].有色金属科学与工程, 2016, 7(3):29-34. http://ysjskx.paperopen.com/oa/DArticle.aspx?type=view&id=2016030006 [28] 吴志颖.含氟稀土精矿焙烧过程中氟的化学行为研究[D].沈阳: 东北大学, 2008: 69-77. [29] SEO W G, ZHOU D, HAMANO T, et al. Calulation of phase diagrams for the CaO-CaF2 and CaO-BaO binary systems by using molecular dynamics[C]//VII International Conference on Molten Slags Fluxes and Salts (Edition: The South African Institute of Mining and Metallurgy), 2004: 835-838.
[30] BALIGIDAD R G, RADHAKRISHNA A. Processing of Fe-Al-C-Ce alloys through air induction melting with flux cover (AIMFC) and electroslag remelting(ESR)[J].Journal of Materials Science, 2002, 37(23):5021-5028. doi: 10.1023/A:1021035615317
[31] REN Y, ZHANG L, YU L, et al. Yield of Y, La, Ce in high temperature alloy during electroslag remelting process[J]. Metallurgical Research & Technology, 2016, 113(4):405. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=962a31c5835bed202c419ea2823e95e1
-
期刊类型引用(4)
1. 李世森,陈宇新,臧喜民,杨杰,孔令种. Ce_2O_3对CaF_2-CaO-Al_2O_3-MgO电渣重熔渣系熔化行为的影响. 钢铁. 2024(03): 117-128 .  百度学术
百度学术
2. 王飞,牛家振,郭盛琦,王俊利,郭靖. 氩气保护电渣重熔脱硫预测研究. 有色金属科学与工程. 2024(04): 487-496 .  本站查看
本站查看
3. 赵博,吴伟,智建国,宿成,武利平. CeO_2含量对CaO-Al_2O_3精炼渣熔化性能的影响. 中国稀土学报. 2023(05): 975-985 .  百度学术
百度学术
4. 王振虎,李彬,郭汉杰,郭靖. 电热合金钢0Cr21Al6NbRE电渣重熔用渣的研究. 特殊钢. 2020(01): 6-11 .  百度学术
百度学术
其他类型引用(3)



 下载:
下载: