Study on biosorption characteristics of spirulina to rare earth ytterbium ions in simulated mine wastewater
-
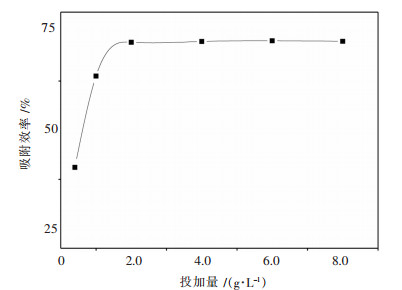
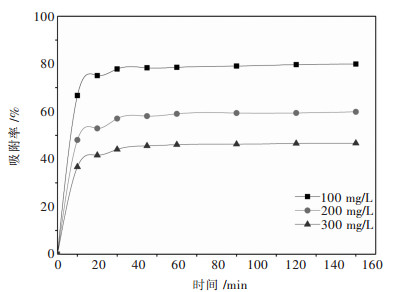
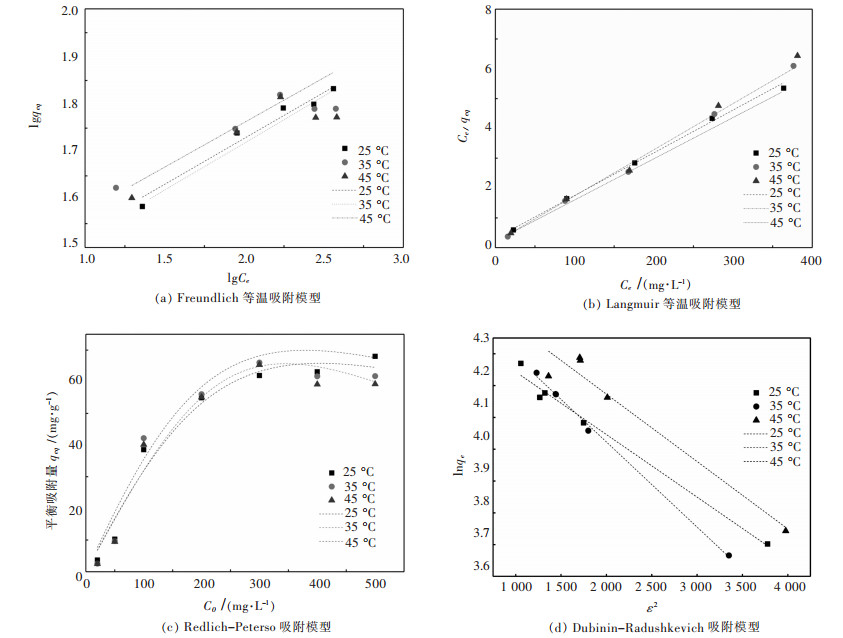
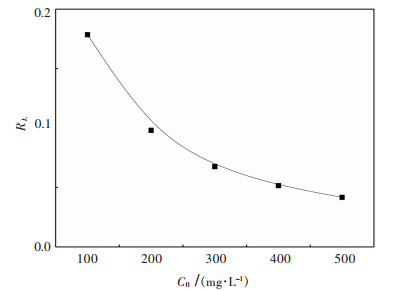
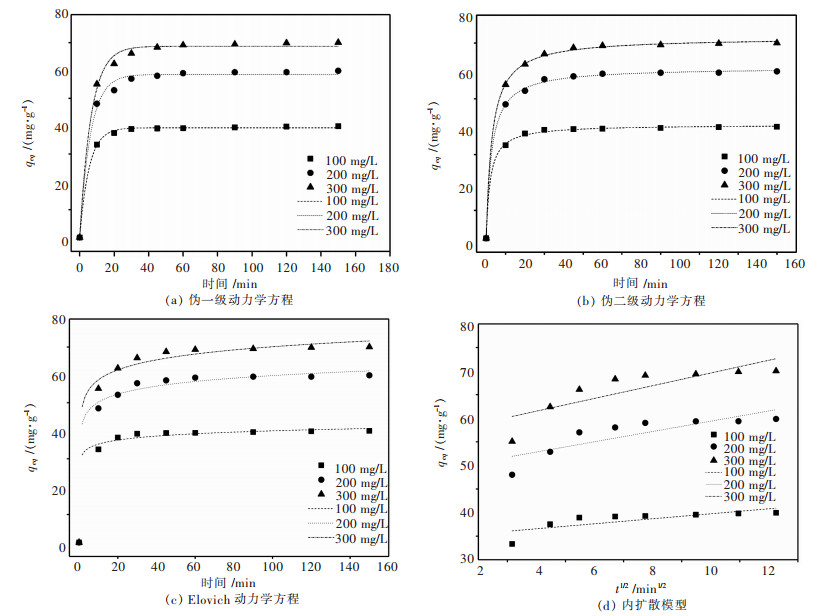
摘要: 通过Zarrouk培养液获得了螺旋藻,并以其为生物质吸附剂,对模拟矿山废水中稀土镱离子的吸附性能进行研究.通过单道扫描电感耦合等离子体发射光谱(ICP-AES)、扫描电镜(SEM)、傅里叶红外光谱仪(FTIR)、多功能成像电子能谱仪(XPS)等分析方法对螺旋藻的结构和吸附性能进行研究.通过Freundlich,Langmuir,Redlich-Peterso和Dubinin-Radushkevich等温吸附模型,以及伪一级、伪二级、Elovich方程和颗粒内扩散动力学模型,对该过程的吸附动力学和热力学规律进行探讨,以了解该吸附过程的机理.结果表明:当被处理液的pH值为5、螺旋藻的剂量为2.0 g/L、初始镱离子浓度为100 mg/L和吸附时间为60 min时,螺旋藻对模拟矿山废水中的稀土镱离子的吸附去除率为77 %,且解吸附率可达到92.3 %,表明螺旋藻的吸附速率快、吸附效果较为理想.研究表明:该过程的吸附动力学行为符合伪二级动力学模型(R2>0.99),主要受化学吸附控制;且吸附等温线能较好用Langmuir方程进行模拟(R2>0.99),属于自发吸热型吸附过程.Abstract: Spirulina was obtained from Zarrouk culture solution and was used as a biomass adsorbent to study the adsorption performance of rare earth cerium ions in simulated mine wastewater. By the means of single channel sequence scanning inductively coupled plasma atomic emission spectrometric(ICP-AES), scanning electron microscope (SEM), fourier transform infrared spectrometer (FTIR), X-ray photoelectron spectroscopy (XPS), the structure and adsorption properties of spirulina was studied. The adsorption kinetics and thermodynamic laws were discussed from the adoption of Freundlich, Langmuir, Redlich-Peterso, Dubin-Radushkevich isothermal adsorption model and pseudo-first-order, pseudo-second-order, elovich equation and intra-particle diffusion model, which can be expected to understand the mechanism of the adsorption process. The adsorption results suggest that the removal rate and the desorption rate of Yb3+ from simulated mine wastewater can reach 77 % and 92.3 % respectively when pH of the treated liquid was 5, dosage of spirulina 2.0 g/L, initial concentration of ytterbium ions 100 mg/L and adsorption time 60 min. In this case, the data indicate that the adsorption rate of spirulina was fast and the adsorption effect was ideal. The adsorption kinetics was well accorded with pseudo-second-order kinetic model (R2>0.99). And the adsorption process was mainly controlled by chemical adsorption. The adsorption isotherm was well simulated by Langmuir equation (R2>0.99). The adsorption of Yb3+ from simulated mine wastewater by spirulina was a spontaneous endothermic process.
-
0 引言
稀土是国防和航天不可或缺的战略性资源.全球范围内,美国稀土储量占全球稀土储藏量的40 %,俄罗斯占30 %,中国占18 %,印度占7 %.我国稀土资源总量的98 %分布在内蒙古的白云鄂博、江西赣南、广东粤北、四川凉山等地区.江西赣南地区的稀土元素以离子形态吸附在花岗岩风化壳的全风化层的黏土当中,由于过去采用的堆浸法的“搬山运动”对环境破坏严重,现在多采用原地浸矿法开采[1].
原地浸矿法的主要特征是,矿石处于天然状态下未经任何位移,通过注液工程往矿层注入溶浸液,使之与矿石中的有用成分接触,进行化学反应[2].赣南稀土矿区的地层构造依次为表土层、含稀土元素的透水全风化层、不透水的基岩,赣南地区的稀土资源非常适合此种开采方法.注液工程主要有打穿表土层的注液孔,开采过程就是通过管道向注液孔里注入溶浸液,然后收集浸出液.但随着注液的进行,注入的液体不仅带走了稀土,还带走了半风化层的细小颗粒,改变了土体的级配,使土体的孔隙和裂缝加大,改变了土体的内摩擦角和黏聚力.注入的液体会造成2个方面的结果,一是溶浸液进入这些孔隙和裂缝,降低土坡的抗剪强度;二是注入的液体补给地下水,使地下水位抬高,减小滑动面的有效法向应力,渗透压力增大,这些都可能致使边坡更容易滑动[3].滑坡造成严重的经济损失和人员伤亡,归因于对滑坡的发生的地点、时间和强度的无法事先预测,所以对赣南稀土矿边坡的监测尤为重要[4-6]!
1 监测方案的构建
1.1 实验场地的概况
江西省龙南县关西2A车间防滑坡实验矿块位于江西省龙南县城东南10 km,行政属于龙南县关西镇管辖.矿体由中部往四周倾斜,沿山脊矿体倾斜较缓,一般为5°~10°.沿山坡矿体倾斜较陡,多数为20°~30°,坡角矿体局部达40°.矿体平均厚度为8.1 m,矿区为似层状面型表露矿体,形态较为简单,其产状和厚度变化明显受地貌形态的制约.
1.2 监测方案构建
采用三维数值模拟,建立水平投影面积为200 m×180 m三维立体模型,找出三维边坡最容易失稳的区域,把该区域作为重点监测区域.对三维模型进行以下处理:①将三维边坡简化为均质三维模型.由于影响边坡稳定性的最敏感地质层为土层,选择该均质边坡为全风化层均质土坡.②简化边界条件和相关参数.③忽略地下水的影响.给模型添加底部和四周位移约束;为了使边坡模型失稳更易显现,相关参数按照表 1的全风化层参数.
表 1 土层计算参数Table 1. Soil calculation parameters弹性模量/GPa 剪切模量/GPa 重度/kN·m-3 内摩擦角/(°) 黏聚力/kPa 0.5 0.2 18 15 18 图 1为利用FLAC3D按照均质边坡参数得到的塑性区[7].shear-p区域已经连成片,一般认为形成了贯通的塑性区是边坡区域失稳的标志,将该区域视为边坡重点监测区域,测线及测孔布置如图 2.
第1条测线布置在K8、K5 2个勘探孔连线的方向上,C2孔埋设2#土压计和C3孔埋设3#土压计,第2条测线布置在K12、K14 2个勘探孔的连线上,C6孔埋设6#上土压计和6#下土压计,在6#监测孔里埋设上测斜仪和下测斜仪.监测系统包含数据从采集、传输、存储,再到处理的流程,配套的子系统包括监测控制中心、综合预警预报等[8].图 3为稀土矿区采场在线监测系统示意图[9].
2 现场试验成果及分析
2.1 土压力监测结果分析
图 4为土压力监测相对值[10].数据显示:2#土压力出现较大异常波动,这可能与埋设时土体未捣实有关;3#监测孔附近坡体平缓,在整个注液过程中基本保持稳定;6#监测孔上部土压力处于稳定缓慢增加的正常状态;6#监测孔下部土压力出现了异常.
2.2 失稳实例分析
6#监测孔位于山坡山脚上部位置,沿山体往下约10 m即是矿区道路.图 5为2014年1月17日清晨坡脚风化岩出现裂缝,阻塞了积液沟,导致积液沟稀土母液沿道路流失,且给矿区道路带来了安全隐患.
1月7日至1月14日期间,6#监测孔下部土压力急剧增大,14日以后增大幅度减小,17日出现坡脚失稳破坏,破坏后应力释放并达到新的平衡,即19日后土压力又趋于稳定.然而,6#监测孔上部土压力基本无变化,说明坡体位移主要发生在下部,但土压力缓慢增大说明山体总体上仍然向下移动,且土压力的监测表明失稳位置出现在全风化层.
图 6为6#监测孔测斜仪时间挠度曲线,图 6中1/0.6 m表示第1个应变计距离地表 0.6 m位置[11].假定测斜管底部不变的挠度数据表明,测斜管底部与第2、3个应变片的相对变化最为明显,1月15日与1月18日之间,第2个应变计的相对内部位移短时间变化极大,而坡脚失稳正好发生于17日.坡脚破坏导致坡体内应力重新分布并于19日达到新平衡,19日后测斜管监测数据又趋于稳定.
3 灰色预测
3.1 灰色理论简介
灰色预测是通过少量的、不确定的信息,建立数学模型并做出预测的一种预测方法.灰色预测的过程是对原始数据进行累加或累减处理,生成新数据序列,把新序列用到微分方程模型中,由此抵消部分由不确定因素引起的随机误差,增加预测精度,更好地分析和判别变性趋势.灰色预测常用GM(1,1)和GM(n,h)模型.灰色系统模型的精度检验按表 2确定[12-14].
表 2 精度检验Table 2. Accuracy test检验指标 精度等级 好 合格 勉强 不合格 P >0.95 >0.80 >0.70 ≤0.70 C < 0.35 < 0.50 < 0.65 ≥0.65 3.2 预测实例与分析
以6#下土压计7 d数据为例,建立GM(1,1)模型[15],模型时间响应函数为:x(k+1)=-13 410.277 9 exp(-0. 002 5)+134 44.164 1,预测结果如表 3:
表 3 土压实测值与预测值对比Table 3. Comparison of soil pressure between measured and predicted监测周期 监测时间 6#号下土压监测值/kPa 6#号下土压预测值/kPa 残差 1 2014-07-01 33.886 2 33.886 2 0 2 2014-07-02 33.442 9 33.310 4 0.1325 3 2014-07-03 33.100 0 33.227 6 -0.1276 4 2014-07-04 33.034 7 33.145 1 -0.1104 5 2014-07-05 33.092 2 33.062 8 0.0294 6 2014-07-06 33.100 0 32.980 7 0.1193 7 2014-07-07 32.855 6 32.898 7 -0.0431 由表 3可得残差均值及原始数据平均值,它们分别为ε=0.000 014,$\mathop x\limits^{-\left( 0 \right)} $=33.215 9,由此又可得Se2=0.008 997,Sx2=0.100 807.因此,后验差比值为C=0.30 < 0.35,小概率误差${\rm{p = p}}\left\{ {\left| {{\varepsilon ^0}\left( k \right)-\bar \varepsilon } \right.} \right\} < 0.6745$Sx=1>0.95,故预测精度等级为好.
4 结论
1)利用FLAC3D和保守的强度参数进行数值模拟,确定了采场边坡失稳区域,作为重点监测区域,并以此制定了在线监测系统实施方案.通过分析土压计及测斜仪的数据,数据发生急剧变化的时刻与现场大雨导致山体部分失稳的时刻相吻合,现场失稳的山体部分与模拟结果相吻合,表明构建的监测系统应用于稀土矿边坡可靠.
2)结合6#下土压计连续7 d监测值,利用灰色理论建立灰色预测模型,预测结果精度等级为好,可以应用到工程实际,同时也为后续的滑坡预警提供支持.
-
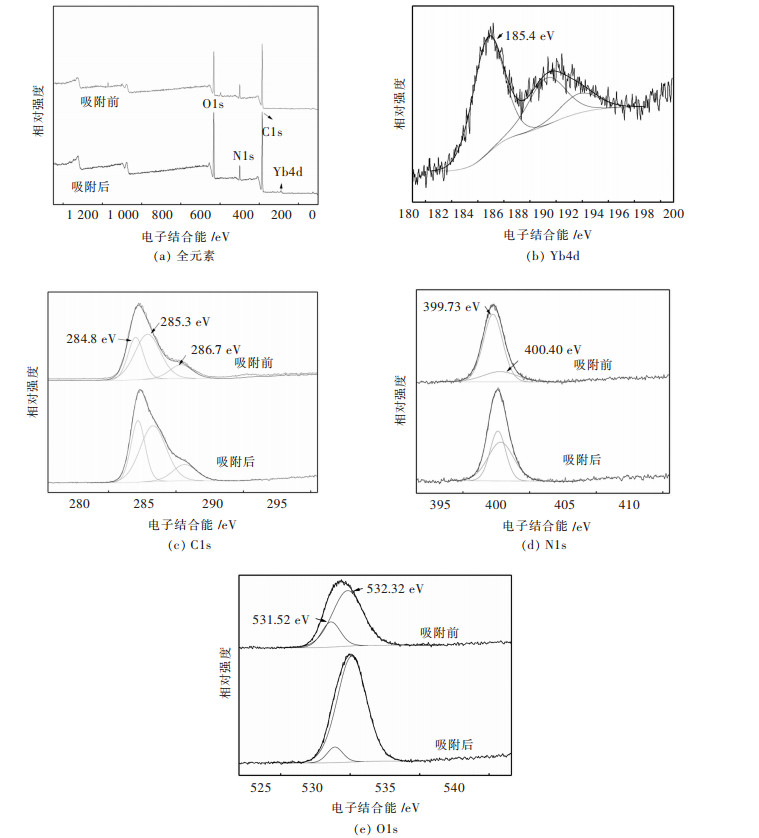
表 1 吸附Yb3+前后螺旋藻中的各元素含量/%
Table 1 Element content of spirulina before and after adsorption of Yb3+ /%

表 2 各等温吸附模型拟合参数数据
Table 2 Fitting data of isothermal adsorption models

表 3 热力学参数数值
Table 3 Thermodynamic parameter values

表 4 动力学方程拟合参数数值
Table 4 Numerical values of fitting parameters of dynamic equations

-
[1] 王岩, 况军.热分析技术的发展现状及其在稀土功能材料中的应用[J].金属功能材料, 2014, 21(4):43-46. http://www.cnki.com.cn/Article/CJFDTotal-JSGC201404016.htm [2] 贺海钧, 赵英.稀土功能材料与其应用产业群之间的关联和趋势分析[J].稀土, 2006, 27(6):91-94. doi: 10.3969/j.issn.1004-0277.2006.06.025 [3] 曹剑锋, 张鹏英, 陈靠山.稀土钕元素的生物学效应及机制研究进展[J].植物生理学报, 2010, 46(4):325-328. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK201000573133 [4] ANNECLAIRE TEXIER, YVES ANDRES A, P L C. Selective biosorption of lanthanide (La, Eu, Yb) Ions by pseudomonas aeruginosa[J]. Environmental Science & Technology, 1999, 33(33):489-495. doi: 10.1021/es9807744
[5] GEOFFREY MICHAEL GADD. Bioremedial potential of microbial mechanisms of metal mobilization and immobilization[J]. Current Opinion in Biotechnology, 2000, 11(3):271-279. doi: 10.1016/S0958-1669(00)00095-1
[6] PHILIP L, IYENGAR L, VENKOBACHAR C. Original papers biosorption of U, La, Pr, Nd, Eu and Dy by pseudomonas aeruginosa[J]. Journal of Industrial Microbiology & Biotechnology, 2000, 25(1):1-7. doi: 10.1038/sj.jim.7000026
[7] XIONG C, YAO C, WANG Y. Sorption behaviour and mechanism of ytterbium(Ⅲ) on imino-diacetic acid resin[J]. Hydrometallurgy, 2006, 82(3):190-194. http://www.sciencedirect.com/science/article/pii/S0304386X06000612
[8] ZHENG Z W, XIONG C H. Adsorption behavior of ytterbium (Ⅲ) on gel-type weak acid resin[J]. Journal of Rare Earths, 2011, 29(5):407-412. doi: 10.1016/S1002-0721(10)60469-3
[9] QIN Q, ZHAO H, YAN L, et al. Extraction of rare earth metals by liquid surfactant membranes containing cyanex272 as a carrier[J]. Mining & Metallurgical Engineering, 2002, 22(3):74-78. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=kygc200203023
[10] MEHTA S K, GAURAUR J P. Use of algae for removing heavy metal ions from wastewater: progress and prospects[J]. Critical Reviews in Biotechnology, 2005, 25(3):113-152. doi: 10.1080/07388550500248571
[11] WANG J L, CHEN C. Biosorbents for heavy metals removal and their future[J]. Biotechnology Advances, 2009, 27(2):195-226. doi: 10.1016/j.biotechadv.2008.11.002
[12] NOURBAKHSH M, SAG Y, öZER D, et al. A comparative study of various biosorbents for removal of chromium(Ⅵ) ions from industrial waste waters[J]. Process Biochemistry, 1994, 29(1):1-5. http://www.sciencedirect.com/science/article/pii/0032959294800529
[13] ÇELEKLI A, BOZKURT H. Bio-sorption of cadmium and nickel ions using spirulina platensis: kinetic and equilibrium studies[J]. Desalination, 2011, 275(1/2/3):141-147. http://www.sciencedirect.com/science/article/pii/S0011916411001809 [14] 张文娟, 谢玲君, 刘鸿. ICP-AES法测定氟碳铈矿中低含量稀土总量[J].有色金属科学与工程, 2016, 7(6):141-146. http://ysjskx.paperopen.com/oa/DArticle.aspx?type=view&id=2016060024 [15] 程明焱, 刘和连, 吴伟明, 等.稀土分析检测方法标准述评[J].有色金属科学与工程, 2012, 3(4):108-114. http://ysjskx.paperopen.com/oa/darticle.aspx?type=view&id=201204019 [16] SöNMEZAY A, ÖNCEL M S, BEKTAÇN. Adsorption of lead and cadmium ions from aqueous solutions using manganoxide minerals[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(12):3131-3139. doi: 10.1016/S1003-6326(12)61765-8
[17] QIU H, LU L V, PAN B C, et al. Critical review in adsorption kinetic models[J]. Journal of Zhejiang University-Science A(Applied Physics & Engineering), 2009, 10(5):716-724. http://d.old.wanfangdata.com.cn/Periodical/zjdxxb-e200905013
[18] 郭钟群, 金解放, 王观石, 等.风化壳淋积稀土矿浸取动力学基础理论研究[J].有色金属科学与工程, 2017, 8(5):127-132. http://ysjskx.paperopen.com/oa/darticle.aspx?type=view&id=2017050019 [19] REZAEI H. Biosorption of chromium by using spirulina, sp[J]. Arabian Journal of Chemistry, 2016, 9(6):846-853. doi: 10.1016/j.arabjc.2013.11.008
[20] HANG T T, VU N D, MATSUKAWA M, et al. Heavy metal biosorption from aqueous solutions by algae inhabiting rice paddies in Vietnam[J]. Journal of Environmental Chemical Engineering, 2016, 4(2):2529-2535. doi: 10.1016/j.jece.2016.04.038
[21] GUIZA S. Biosorption of heavy metal from aqueous solution using cellulosic waste orange peel[J]. Ecological Engineering, 2017, 99:134-140. doi: 10.1016/j.ecoleng.2016.11.043
[22] DENIZ F, KARABULUT A. Biosorption of heavy metal ions by chemically modified biomass of coastal seaweed community: Studies on phycoremediation system modeling and design[J]. Ecological Engineering, 2017, 106:101-108. doi: 10.1016/j.ecoleng.2017.05.024
[23] HE J, CHEN J P. A comprehensive review on biosorption of heavy metals by algal biomass: materials, performances, chemistry, and modeling simulation tools.[J]. Bioresource Technology, 2014, 160(6):67-78. http://www.sciencedirect.com/science/article/pii/S0960852414000935
[24] REZAEI H. Biosorption of chromium by using spirulina, sp[J]. Arabian Journal of Chemistry, 2016, 9(6):846-853. doi: 10.1016/j.arabjc.2013.11.008
[25] AZIMI G, DHIMAN R, KWON H M, et al. Hydrophobicity of rare-earth oxide ceramics[J]. Nature Materials, 2013, 12(4):315-320. doi: 10.1038/nmat3545
[26] SHU Q, TANG G, LESMANA H, et al. Preparation, characterization and application of a novel solid Brönsted acid catalyst SO42- /La3+ /C for biodiesel production via esterification of oleic acid and methanol[J]. Renewable Energy, 2018, 119:253-261. doi: 10.1016/j.renene.2017.12.013
[27] LIU B, HUANG Y. Polyethyleneimine modified eggshell membrane as a novel biosorbent for adsorption and detoxification of Cr(Ⅵ) from water[J]. Journal of Materials Chemistry, 2011, 21(43):17413-17418. doi: 10.1039/c1jm12329g
[28] HUANG L Z, ZENG G M, HUANG D L, et al. Adsorption of lead(Ⅱ) from aqueous solution onto hydrilla verticillata[J]. Biodegradation, 2009, 20(5):651-660. doi: 10.1007/s10532-009-9252-4



 下载:
下载: