Thermodynamic analysis on removing Cr(III) by phosphate precipitation
-
摘要: 除铬是含铬电镀污泥湿法冶金过程重要步骤.针对磷酸盐沉淀法从溶液中净化除铬过程进行热力学分析,绘制了25 ℃时Me-P-H2O(Me: Cr(III), Zn(II), Cu(II), Fe(II), Fe(III), Ni(II))系组浓度对数-pH图,利用热力学平衡图对磷酸盐沉淀法从含铁等金属元素中净化除铁和磷酸铬碱分解过程进行热力学分析.结果表明,pH值为1.0 ~ 5.0磷酸盐形成由易至难依次为Cr(III)>Fe(III) >Fe(II)>Ni(II)>Cu(II)>Zn(II); 磷酸盐沉淀法难以有效地将Cr(III)与Fe(III)分离,而可分离Cr(III)和Fe(II),且较优pH约为2;整个pH值范围Me-P-H2O系可以分为难溶磷酸盐稳定区、Me(OH)n稳定区; 高pH区磷酸盐中的Me转变为稳定的Me(OH)n,实现磷酸盐碱分解.验证实验表明,加入1.1倍理论量的磷酸钠,控制沉淀pH值为2.0,铬、铁、锌、铜、镍沉淀率分别为94.12 %、5.51 %、0.33 %、0.22 %、0.34 %; 氢氧化钠分解磷酸铬时,磷、铬浸出率分别为90.63 %、5.10 %,实现磷铬有效分离.实验与理论基本相符.Abstract: Cr (III) removal is an important step in the hydrometallurgical process of chromium electroplating sludge. After the thermodynamics analysis of chromium removal through phosphate precipitation, the logarithm concentration-pH diagram of Me-P-H2O (Me: Cr(III), Zn(II), Cu(II), Fe(II), Fe(III), Ni(II)) at 25 ℃was drawn. Based on this thermodynamic equilibrium diagram, thermodynamic analysis was carried out to discuss the removal of iron from metal elements and the decomposition process of chromium phosphate sodium hydroxide. The results show that the formation of phosphate with pH value from 1.0 to 5.0, from easy to difficult, is in the following order: Cr(III) > Fe(III) > Fe(II) > Ni(II) > Cu(II) > Zn(II). It is difficult to separate Cr (III) from Fe (III) effectively by phosphate precipitation method, but Cr(III) and Fe(II) can be separated by it, and the best pH value is 2. The scope of the whole pH value of Me-P-H2O can be divided into the stable zone of sparingly soluble phosphate, and that of Me(OH)n. With the transformation of Me in the high pH zone of phosphate into stable Me(OH)n, the decomposition of phosphate base was achieved. The verification experiment results show that when added 110 % theoretical amounts of sodium phosphate and the pH is 2.0, the precipitation rate of Cr, Fe, Zn, Cu, Ni is 94.12 %, 5.51 %, 0.33 %, 0.22 % and 0.34 % respectively. The leaching rate of phosphorus and chromium is 90.63 % and 5.10 % respectively when the chromium phosphate is decomposed by sodium hydroxide, which is consistent with the thermodynamic analysis.
-
Keywords:
- phosphate /
- chromium /
- iron /
- precipitation /
- thermodynamic
-
铬是重要的金属资源,广泛应用于钢铁、电镀、皮革、铸造等领域[1-3].铬的某些化合物具有较强的毒性,如Cr(VI)化合物已被国际癌症研究中心(IARC)确定为人类致癌物[4].在铬化学物的生产和应用过程常常产生含铬废料,尤其是电镀行业产生的含铬废水,是目前最大的铬污染源之一[5-6].电镀含铬废水目前多通过石灰、氢氧化钠等中和处理,将重金属富集在含铬电镀污泥中[7-8].采用无机酸或生物浸出等方式[9-11]将电镀污泥的重金属溶解至溶液中,再从溶液中分离回收金属是一种益于资源综合利用和环境保护的方法.然而浸出液成分复杂多含有铬与铁、铜、镍、锌等金属元素.目前,从酸性溶液中分离铬与铁、铜、镍、锌的方法主要包括:溶剂萃取法和沉淀法.酸性磷类萃取剂D2EHPA优先萃取Fe(III)而分离Cr(III),但负载有机相中Fe(III)需要高浓度盐酸才能反萃[12];P204、P507能够优先萃取Cr(III)与镍、锌分离,负载有机相中铬难以被反萃[13-14].针铁矿沉淀法[15]能有效除去铁,但铬损失较高,草酸亚铁沉淀[16]、莫尔盐沉淀[17]、铁蓝沉淀法[18]均较好的实现了铬与铁的分离,但消耗了较为昂贵的沉淀剂,且铬仍存在于溶液中,需要再将铬与其他金属分离.杨振宁等[19]研究表明磷酸盐沉淀法能够选择性沉淀Fe(III)、Cr(III)、Al(III)与镍分离;徐志峰[20]等将溶液中Fe(III)还原为Fe(II),采用磷酸盐选择性沉淀Cr(III)实现铬铁分离;郭峰[21]将磷酸铬渣通过碱分解实现铬的富集和磷的回用,降低了生产成本,显然磷酸盐沉淀法从含铬溶液中回收铬具有显著的优势.
目前,针对含铬电镀污泥浸出液,采用磷酸盐沉淀法实现铬与金属分离(铁、铜、镍、锌)的热力学研究报道较少,文中拟以磷酸盐沉淀铬工艺处理含铬电镀污泥浸出液的除铬和磷回收过程的热力学进行研究.含铬电镀污泥浸出液中主要的金属元素(Me)包括Cr(III)、Zn(II)、Fe(II)、Fe(III)、Cu(II)、Ni(II).通过热力学计算,依次绘制对应条件下的热力学平衡图,并对结果进行分析,以期能够深化对过程的认知,并对磷酸盐除铬工艺提供理论指导和实验验证.
1 热力学计算与验证实验方法
1.1 热力学计算方法
Men+-P-H2O系中,溶液中可能存在的物质包括H+、OH-、Men+、Me(OH)mn-m、PO43-、HPO42-、H2PO4-、H3PO4.体系可能存在的沉淀物包括CrPO4、Zn3(PO4)2、Cu3(PO4)2、Ni3(PO4)2、Fe3(PO4)2、FePO4、Cr(OH)3、Zn(OH)2、Cu(OH)2、Ni(OH)2、Fe(OH)3、Fe(OH)2.溶液中可能存在的平衡对应的平衡常数(K)以及计算关系列于表 1[22].
表 1 Men+-P-H2O体系化学反应及其平衡常数(298K)Table 1. Equilibrium reactions and constants for Men+-P-H2O system at 298 K序号 反应式 log K 计算关系式 1 Cr3++OH-=CrOH2+ 10.1 [CrOH2+]=[Cr3+][OH-]×1010.1 2 Cr3++OH-=CrOH2+ 17.8 [Cr(OH)2+]=[Cr3+][OH-]2×1010.1 3 Cr3++4OH-=CrOH4- 29.9 [Cr(OH)4-]=[Cr3+][OH-]4×1029.9 4 Cu2++OH-=CuOH+ 7.00 [CrOH+]=[Cr2+][OH-]×107 5 Cr2++2OH-=Cu(OH)2(a) 13.68 [Cu(OH)2(a)]=[Cu2+][OH-]2×1013.68 6 Cu2++3OH-=Cu(OH)3- 17.00 [Cu(OH)3-]=[Cu2+][OH-]3×1017 7 Cr2++4OH-=Cu(OH)42- 18.50 [Cu(OH)42-]=[Cu2+][OH-]4×1018.5 8 Fe2++OH-=FeOH+ 5.56 [FeOH+]=[Fe2+][OH-]×105.56 9 Fe2++2OH-=Fe(OH)2(a) 9.77 [Fe(OH)2(a)]=[Fe2+][OH-]2×109.77 10 Fe2++3OH-=Fe(OH)3- 9.67 [Fe(OH)3-]=[Fe2+][OH-]3×109.67 11 Fe2++4OH-=Fe(OH)42- 8.58 [Fe(OH)42-]=[Fe2+][OH-]4×108.58 12 Fe3++OH-=FeOH2+ 11.87 [FeOH2+]=[Fe3+][OH-]×1011.87 13 Fe3++OH-=FeOH2+ 21.17 [FeOH2+]=[Fe3+][OH-]2×1021.17 14 Fe3++3OH-=FeOH3(a) 29.67 [FeOH3(a)]=[Fe3+][OH-]3×1029.67 15 Ni2++OH-=NiOH+ 4.97 [NiOH+]=[Ni2+][OH-]×104.97 16 Ni2++2OH-=Ni(OH)2(a) 8.55 [Ni(OH)2(a)]=[Ni2+][OH-]2×108.55 17 Ni2++3OH-=Ni(OH)3- 11.33 [Ni(OH)3-]=[Ni2+][OH-]3×1011.33 18 Ni2++OH-=ZnOH+ 4.40 [ZnOH+]=[Zn2+][OH-]×104.40 19 Zn2++2OH-=Zn(OH)2(a) 11.30 [Zn(OH)2(a)]=[Zn2+][OH-]2×1011.30 20 Zn2++3OH-=Zn(OH)3- 14.14 [Zn(OH)3-]=[Zn2+][OH-]3×1014.14 21 Zn2++4OH-=Zn(OH)42- 17.66 [Zn(OH)42-]=[Zn2+][OH-]4×1017.66 22 H++OH-=H2O 14.00 [H+][OH-]=10-14 23 H++PO43-=HPO42- 12.36 [PHO42-]=[PO43-][H+]4×1012.36 24 H++HPO42-=H2PO4- 7.20 [H2PO4-]=[HPO42-][H+]4×107.2 25 H++H2PO4-=H3PO4 2.04 [H3PO4]=[H2PO4-][H+]4×102.04 26 Cr(OH)3(S)=Cr3++3OH- -30.2 [Cr3+][OH-]3=10-30.2 27 Cu(OH)2(S)=Cu2++2OH- -19.66 [Cu2+][OH-]2=10-19.66 28 Fe(OH)2(S)=Fe2++2OH- -16.31 [Fe2+][OH-]2=10-16.31 29 Fe(OH)2(S)=Fe3++3OH- -38.55 [Fe3+][OH-]3=10-38.55 30 Ni(OH)2(S)=Ni2++2OH- -15.26 [Ni2+][OH-]2=10-15.26 31 Zn(OH)2(S)=Zn2++2OH- -16.50 [Zn2+][OH-]2=10-16.5 32 CrPO4(S)=Cr3++PO43- -22.62 [Cr3+][PO43-]2=10-22.62 33 Cu3(PO4)2(S)=3Cu2++2PO43- -36.85 [Cu2+]3[PO43-]2=10-36.85 34 Fe3(PO4)2(S)=3Fe2++2PO43- -36.85 [Fe2+]3[PO43-]2=10-36.85 35 FePO4(S)=Fe2++PO43- -23.0 [Fe3+][PO43-]=10-23.0 36 Ni3(PO4)2(S)=3Ni2++2PO43- -31.32 [Ni2+]3[PO43-]2=10-31.32 37 Zn3(PO4)2(S)=3Zn2++2PO43- -32.04 [Zn2+]3[PO43-]2=10-32.04 表 1中式(1)~式(25) 表示体系中离子的平衡,式(26)~式(37) 表示体系的固体物质的理解平衡.设[Me]T、[P]T分别为溶液中游离Me、P总浓度,“[]”为溶液中各游离组分的浓度,由于计算缺乏相关的物质活度系数,故本研究计算过程以浓度代替活度.根据质量守恒定律,溶液中Me、P总量如下:
$$ {{\rm{[Cr(III)]}}_{\rm{T}}}{\rm{ = [C}}{{\rm{r}}^{{\rm{3 + }}}}{\rm{] + [CrO}}{{\rm{H}}^{{\rm{2 + }}}}{\rm{] + [Cr(OH}}{{\rm{)}}_{\rm{2}}}^{\rm{ + }}{\rm{] + [Cr(OH}}{{\rm{)}}_{\rm{4}}}^{\rm{-}}{\rm{]}} $$ (38) $$ \begin{array}{l} {{\rm{[Cu(II)]}}_{\rm{T}}}{\rm{ = [C}}{{\rm{u}}^{{\rm{2 + }}}}{\rm{] + [CuO}}{{\rm{H}}^{\rm{ + }}}{\rm{] + [Cu(OH}}{{\rm{)}}_{{\rm{2(a)}}}}{\rm{] + }}\\ \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;{\rm{[Cu(OH}}{{\rm{)}}_{\rm{3}}}^{\rm{-}}{\rm{] + [Cu(OH}}{{\rm{)}}_{\rm{4}}}^{{\rm{2-}}}{\rm{]}} \end{array} $$ (39) $$ \begin{array}{l} {{\rm{[Fe(II)]}}_{\rm{T}}}{\rm{ = [F}}{{\rm{e}}^{{\rm{2 + }}}}{\rm{] + [FeO}}{{\rm{H}}^{\rm{ + }}}{\rm{] + [Fe(OH}}{{\rm{)}}_{{\rm{2(a)}}}}{\rm{] + }}\\ \;\;\;\;\;\;\;\;\;\;\;\;\;\;{\rm{[Fe(OH}}{{\rm{)}}_{\rm{3}}}^{\rm{-}}{\rm{] + [Fe(OH}}{{\rm{)}}_{\rm{4}}}^{{\rm{2-}}}{\rm{]}} \end{array} $$ (40) $$ {{\rm{[Fe(III)]}}_{\rm{T}}}{\rm{ = [F}}{{\rm{e}}^{{\rm{3 + }}}}{\rm{] + [FeO}}{{\rm{H}}^{{\rm{2 + }}}}{\rm{] + [Fe(OH}}{{\rm{)}}_{\rm{2}}}^{\rm{ + }}{\rm{] + [Fe(OH}}{{\rm{)}}_{{\rm{3(a)}}}}{\rm{] }} $$ (41) $$ {{\rm{[Ni(II)]}}_{\rm{T}}}{\rm{ = [N}}{{\rm{i}}^{{\rm{2 + }}}}{\rm{] + [NiO}}{{\rm{H}}^{\rm{ + }}}{\rm{] + [Ni(OH}}{{\rm{)}}_{{\rm{2(a)}}}}{\rm{] + [Ni(OH}}{{\rm{)}}_{\rm{3}}}^{\rm{-}}{\rm{]}} $$ (42) $$ \begin{array}{l} {{\rm{[Zn(II)]}}_{\rm{T}}}{\rm{ = [Z}}{{\rm{n}}^{{\rm{2 + }}}}{\rm{] + [ZnO}}{{\rm{H}}^{\rm{ + }}}{\rm{] + [Zn(OH}}{{\rm{)}}_{{\rm{2(a)}}}}{\rm{] + }}\\ \;\;\;\;\;\;\;\;\;\;\;\;\;\;\;{\rm{[Zn(OH}}{{\rm{)}}_{\rm{3}}}^{\rm{-}}{\rm{] + [Zn(OH}}{{\rm{)}}_{\rm{4}}}^{{\rm{2-}}}{\rm{]}} \end{array} $$ (43) $$ {{\rm{[P]}}_{\rm{T}}}{\rm{ = [P}}{{\rm{O}}_{\rm{4}}}^{{\rm{3-}}}{\rm{] + [HP}}{{\rm{O}}_{\rm{4}}}^{{\rm{2-}}}{\rm{] + [}}{{\rm{H}}_{\rm{2}}}{\rm{P}}{{\rm{O}}_{\rm{4}}}^{\rm{-}}{\rm{] + [}}{{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{4}}}{\rm{]}} $$ (44) 根据同时平衡,可以将表 1中的式(1)~式(25) 分别代入式(38)~式(44),则[Me]T只与[Men+]、[OH-]、[PO43-]的量相关.水相[Men+]与对应氢氧化物、磷酸盐沉淀形成离解平衡,已知[P]T和溶液pH求解得到对应的[PO43-]、[OH-]代入式(26)~式(37) 即可求解对应[Men+]值,最终求解得出[Me]T.
1.2 验证实验方法
实验所采用的试剂均为分析纯,水为去离子水.采用硫酸盐和稀硫酸配制得到料液成分:Cr(III)、Fe(II)、Zn(II)、Cu(II)、Ni(II)浓度分别为5.63 g/L、3.55 g/L、2.51 g/L、3.55 g/L、2.13 g/L,pH值为1.0.以5.5 mol/L的磷酸钠溶液为沉淀剂,4.5 mol/L的NaOH或H2SO4调节溶液的pH值.磷酸盐沉淀实验是考察溶液pH值对沉淀的影响,实验在搅拌条件下缓慢的加入1.1倍沉淀铬理论量的磷酸钠溶液,控制溶液温度为85 ℃,过程保持溶液pH值稳定,加料完毕后继续搅拌1.0 h,然后分析溶液中金属含量,计算金属的沉淀率.
将磷酸铬(Cr 13.08 %, P 7.02 %)与不同氢氧化钠浓度的溶液按照液固比VL/WS=5 mL/g,在85 ℃反应1.0 h,反应结束后分析溶液P、Cr、NaOH浓度.计算磷、铬的浸出率.
实验溶液中金属元素含量采用ICP-AES分析,溶液中NaOH浓度采用酸碱滴定法测试.
2 结果与讨论
对Men+-P-H2O体系的热力学进行研究,以论证磷酸盐法对含铬电镀污泥酸性浸出液除铬和磷酸铬渣回收磷的原理;研究溶液中Me、P含量和pH值变化对磷酸盐沉淀形成的影响,碱用量对磷酸铬分解的影响;通过实验对理论研究结果进行验证.
2.1 Men+-H2O、Men+-P-H2O系比较
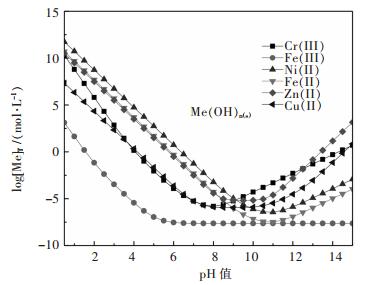
图 1所示为金属水解沉淀时溶液[Me]T与pH值的关系.结果表明金属离子随着溶液pH值增加水解难易顺序依次为:Fe(III)>>Cu(II)≈Cr(III)>Zn(II)≈Fe(II)>Ni(II).结果表明,Fe(III)能够优先水解与Cr(III)分离.而实际操作时,铁水解沉淀过程造成大约30 %铬的损失[22-23],因此铬铁分离效果不理想.
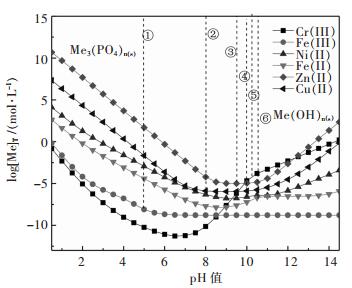
图 2所示为金属磷酸盐沉淀时[Me]T与pH值的关系,虚线① ~ ⑥ 表示对应磷酸盐与氢氧化物的转化pH值.结果表明:pH为1.0 ~ 5.0时,金属磷酸盐沉淀顺序依次为Cr(III) > Fe(III) > Fe(II) > Ni(II) > Cu(II) > Zn(II);pH值增加金属磷酸盐转变为对应的氢氧化物,Fe(III)、Cu(II)、Zn(II)、Ni(II)、Cr(III)、Fe(II)的磷酸盐与氢氧化物之间转化的pH值分别为5.0、8.0、9.5、10.0、10.2、10.5.显然磷酸盐沉淀法可以从含Ni、Zn、Cu溶液中除铁铬,CrPO4能够被碱分解,生成Cr(OH)3,同时再生成磷酸盐.
2.2 磷酸盐沉淀法分离Cr(III)与Fe(III)/Fe(II)
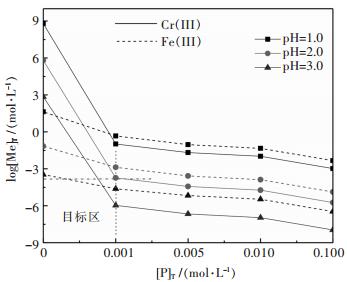
图 3所示,随着[P]T、pH值的增加,溶液中金属浓度降低;为了高效沉淀铬([Cr]T≤10-4 mol/L)和减少溶液中磷的残留([P]T≤0.001 mol/L),要求沉淀过程溶液pH≥2.0,而此pH时溶液中Fe(III)浓度低于10-2.8 mol/L.显然,由于Fe(III)、Cr(III)形成磷酸盐差异性较少,磷酸盐沉淀法难以实现Cr(III)与Fe(III)有效分离.
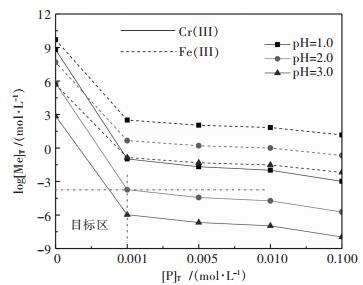
图 4所示,随着溶液中磷和pH值的增加,铁、铬浓度降低,且Cr(III)的浓度低于Fe(II),说明Cr(III)的氢氧化物和磷酸盐的沉淀均优先于Fe(II)形成;溶液pH为2.0、[P]T为0.001 mol/L时,溶液中[Cr(III)]T约为10-4 mol/L,而[Fe(II)]T约为100.66 mol/L,此时具有良好的铬铁分离性能;当pH为3.0,[P]T0.001 mol/L时,溶液中[Cr]T约为10-6.0 mol/L,而[Fe(II)]T约为10-0.99mol/L,沉淀时容易造成铁的沉淀,不利于铬铁分离.显然磷酸盐沉淀法具有较强的Cr(III)、Fe(II)分离能力,且较佳的pH约为2.0.
2.3 NaOH分解CrPO4
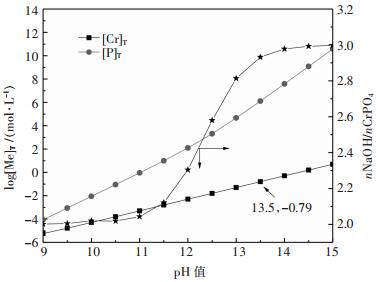
图 5所示为NaOH加入量对CrPO4分解的影响,其中nNaOH/nCrPO4表示NaOH加入量与CrPO4物质量的倍数,[P]T、[Cr]T分别表示溶液平衡磷、铬浓度.结果表明,随着nNaOH/nCrPO4的增加,溶液平衡pH值和磷、铬浓度均增大;同等pH值下磷的浓度远大于铬,说明分解磷易进入溶液中,而铬留在固相中,当溶液pH为13.5时,溶液中铬浓度达到10-0.79 mol/L,再增加碱度则易于造成铬进入溶液中增加,因此需要控制浸出液碱度,以减少铬的浸出率.结果说明磷酸铬能够被氢氧化钠分解,磷进入溶液中,铬主要保留在固相中,实现磷的再生和铬渣的富集.
2.4 验证实验
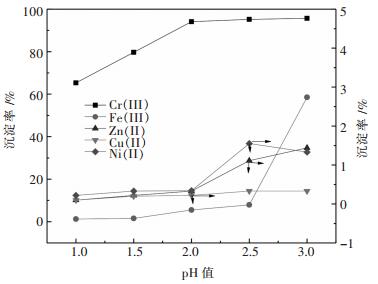
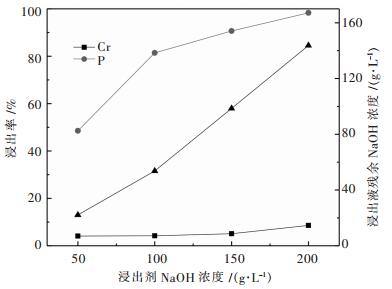
图 6所示表明磷酸盐对铬的沉淀率远高于其他金属元素,金属沉淀率均随着pH值增加而增加;当溶液pH为2.0时,铬、铁、锌、铜、镍沉淀率分别为94.12 %、5.51 %、0.33 %、0.22 %、0.34 %,铬与铁、锌、铜、镍之间的分离效果较好. 图 7所示表明磷的浸出率随着浸出剂中氢氧化钠浓度提高而增加,而铬在碱液中浓度(浸出率)也随着浸出液NaOH浓度增加而增加;当浸出液中残余NaOH浓度为98 g/L,对应磷、铬浸出率分别为90.63 %、5.10 %.说明氢氧化钠可以实现磷酸铬的碱分解和铬与磷的分离.验证实验与热力学分析结论基本相符.
3 结论
1) Men+-P-H2O系,pH为1.0~5.0时,金属磷酸盐沉淀顺序依次为Cr(III) > Fe(III) > Fe(II) > Ni(II) > Cu(II) > Zn(II),Fe(III)、Cu(II)、Zn(II)、Ni(II)、Cr(III)、Fe(II)的磷酸盐在较高pH值条件转化为氢氧化物,其对应转化的pH值分别为5.0、8.0、9.5、10.0、10.2、10.5.
2) Fe(III)、Cr(III)形成磷酸盐差异性较少,磷酸盐沉淀法难以实现Cr(III)与Fe(III)有效分离;而磷酸盐沉淀法具有优异的Cr(III)、Fe(II)分离能力,较优的反应pH约为2.0.
3) 磷酸铬被氢氧化钠分解,磷进入溶液中,铬主要保留在固相中,实现磷的再生和铬渣的富集和后续利用.
4) 验证实验表明,沉淀pH=2.0,加入1.1倍理论量的磷酸钠,铬选择性沉淀与铁镍铜锌有效分离;氢氧化钠分解磷酸铬时,磷、铬浸出率分别为90.63 %、5.10 %,实现磷铬有效分离.
-
表 1 Men+-P-H2O体系化学反应及其平衡常数(298K)
Table 1 Equilibrium reactions and constants for Men+-P-H2O system at 298 K
序号 反应式 log K 计算关系式 1 Cr3++OH-=CrOH2+ 10.1 [CrOH2+]=[Cr3+][OH-]×1010.1 2 Cr3++OH-=CrOH2+ 17.8 [Cr(OH)2+]=[Cr3+][OH-]2×1010.1 3 Cr3++4OH-=CrOH4- 29.9 [Cr(OH)4-]=[Cr3+][OH-]4×1029.9 4 Cu2++OH-=CuOH+ 7.00 [CrOH+]=[Cr2+][OH-]×107 5 Cr2++2OH-=Cu(OH)2(a) 13.68 [Cu(OH)2(a)]=[Cu2+][OH-]2×1013.68 6 Cu2++3OH-=Cu(OH)3- 17.00 [Cu(OH)3-]=[Cu2+][OH-]3×1017 7 Cr2++4OH-=Cu(OH)42- 18.50 [Cu(OH)42-]=[Cu2+][OH-]4×1018.5 8 Fe2++OH-=FeOH+ 5.56 [FeOH+]=[Fe2+][OH-]×105.56 9 Fe2++2OH-=Fe(OH)2(a) 9.77 [Fe(OH)2(a)]=[Fe2+][OH-]2×109.77 10 Fe2++3OH-=Fe(OH)3- 9.67 [Fe(OH)3-]=[Fe2+][OH-]3×109.67 11 Fe2++4OH-=Fe(OH)42- 8.58 [Fe(OH)42-]=[Fe2+][OH-]4×108.58 12 Fe3++OH-=FeOH2+ 11.87 [FeOH2+]=[Fe3+][OH-]×1011.87 13 Fe3++OH-=FeOH2+ 21.17 [FeOH2+]=[Fe3+][OH-]2×1021.17 14 Fe3++3OH-=FeOH3(a) 29.67 [FeOH3(a)]=[Fe3+][OH-]3×1029.67 15 Ni2++OH-=NiOH+ 4.97 [NiOH+]=[Ni2+][OH-]×104.97 16 Ni2++2OH-=Ni(OH)2(a) 8.55 [Ni(OH)2(a)]=[Ni2+][OH-]2×108.55 17 Ni2++3OH-=Ni(OH)3- 11.33 [Ni(OH)3-]=[Ni2+][OH-]3×1011.33 18 Ni2++OH-=ZnOH+ 4.40 [ZnOH+]=[Zn2+][OH-]×104.40 19 Zn2++2OH-=Zn(OH)2(a) 11.30 [Zn(OH)2(a)]=[Zn2+][OH-]2×1011.30 20 Zn2++3OH-=Zn(OH)3- 14.14 [Zn(OH)3-]=[Zn2+][OH-]3×1014.14 21 Zn2++4OH-=Zn(OH)42- 17.66 [Zn(OH)42-]=[Zn2+][OH-]4×1017.66 22 H++OH-=H2O 14.00 [H+][OH-]=10-14 23 H++PO43-=HPO42- 12.36 [PHO42-]=[PO43-][H+]4×1012.36 24 H++HPO42-=H2PO4- 7.20 [H2PO4-]=[HPO42-][H+]4×107.2 25 H++H2PO4-=H3PO4 2.04 [H3PO4]=[H2PO4-][H+]4×102.04 26 Cr(OH)3(S)=Cr3++3OH- -30.2 [Cr3+][OH-]3=10-30.2 27 Cu(OH)2(S)=Cu2++2OH- -19.66 [Cu2+][OH-]2=10-19.66 28 Fe(OH)2(S)=Fe2++2OH- -16.31 [Fe2+][OH-]2=10-16.31 29 Fe(OH)2(S)=Fe3++3OH- -38.55 [Fe3+][OH-]3=10-38.55 30 Ni(OH)2(S)=Ni2++2OH- -15.26 [Ni2+][OH-]2=10-15.26 31 Zn(OH)2(S)=Zn2++2OH- -16.50 [Zn2+][OH-]2=10-16.5 32 CrPO4(S)=Cr3++PO43- -22.62 [Cr3+][PO43-]2=10-22.62 33 Cu3(PO4)2(S)=3Cu2++2PO43- -36.85 [Cu2+]3[PO43-]2=10-36.85 34 Fe3(PO4)2(S)=3Fe2++2PO43- -36.85 [Fe2+]3[PO43-]2=10-36.85 35 FePO4(S)=Fe2++PO43- -23.0 [Fe3+][PO43-]=10-23.0 36 Ni3(PO4)2(S)=3Ni2++2PO43- -31.32 [Ni2+]3[PO43-]2=10-31.32 37 Zn3(PO4)2(S)=3Zn2++2PO43- -32.04 [Zn2+]3[PO43-]2=10-32.04 -
[1] 任志新.含铬钢铁中铬的炉前分析溶样方法选择[J].冶金分析, 2002, 22(1):71-72. http://www.cnki.com.cn/Article/CJFDTOTAL-YJFX200201028.htm [2] 屠振密, 郑剑, 李宁, 等.三价铬电镀铬现状及发展趋势[J].表面技术, 2007, 36(5):59-63. http://www.cnki.com.cn/Article/CJFDTOTAL-BMJS200705020.htm [3] 苏德强, 王坤余, 琚海燕.用铬革屑制备皮革复鞣填充剂的进展[J].皮革科学与工程, 2007, 17(6):40-43. http://www.cnki.com.cn/Article/CJFDTOTAL-PGKG200706010.htm [4] 吴继明, 程胜高.探讨六价铬对人体健康的影响及防治措施[J].现代预防医学, 2009, 36(24):4610-4611. http://www.cnki.com.cn/Article/CJFDTOTAL-XDYF200924008.htm [5] 李岩, 李亚林, 郑波, 等.含铬电镀废水的资源化处理[J].环境科学与技术, 2009, 32(6):145-148. http://www.cnki.com.cn/Article/CJFDTOTAL-FJKS200906033.htm [6] 蔡玉婷.电镀废水对人体的危害及其集中处理[J].农业环境科学学报, 2010, 29(增刊1):205-208. http://www.cnki.com.cn/Article/CJFDTOTAL-NHBH2010S1041.htm [7] 颜家保, 王朝霞, 吴文升.还原沉淀法处理含铬废水的工艺研究[J].武汉科技大学学报(自然科学版), 2002, 25(1):43-44. http://www.cnki.com.cn/Article/CJFDTOTAL-YEKJ200201012.htm [8] 曾炜, 陈楷翰, 黄妙龄, 等.二氧化硫脲/Ca(OH)2-铁氧体法处理铜铬电镀废水工艺[J].泉州师范学院学报, 2008, 26(4):67-70. http://www.cnki.com.cn/Article/CJFDTOTAL-QZXB200804014.htm [9] 姚倩, 张宇峰, 葛苏苏, 等.硫酸浸出电镀污泥中Zn、Cr条件及其动力学研究[J].安徽农学通报, 2015(7):102-104. http://www.cnki.com.cn/Article/CJFDTOTAL-AHNB201507047.htm [10] 毕文龙, 崔雨琪, 方迪, 等.嗜酸硫杆菌和黑曲霉对电镀污泥重金属浸出效果[J].环境工程学报, 2014, 8(10):4402-4408. http://www.cnki.com.cn/Article/CJFDTOTAL-HJJZ201410059.htm [11] PRABHU S V, BASKAR R. Effects of sulfur concentration and kinetics on heavy metal bioleaching from electroplating sludge using bubble column[J]. Research Journal of Chemistry & Environment, 2014, 18(12):70-76. https://www.researchgate.net/publication/288541870_Effects_of_sulfur_concentration_and_kinetics_on_heavy_metal_bioleaching_from_electroplating_sludge_using_bubble_column
[12] 马宏瑞, 李冬雪, 石季峰.有机膦萃取分离制革污泥淋滤液中Cr和Fe[J].环境化学, 2007, 26(4):508-511. http://www.cnki.com.cn/Article/CJFDTOTAL-HJHX200704021.htm [13] YU KEUNG PETER S, XUE L Z. Extraction of zinc and chromium(III) and its application to treatment of alloy electroplating wastewater[J]. Separation Science and Technology, 2003, 38(2):405-425. doi: 10.1081/SS-120016582
[14] 陈曙生, 黄德贤, 林盛煜, 等. P507萃取分离含镍废水中铬的工艺研究[J].江西冶金, 2002, 22(4):28-30. http://www.cnki.com.cn/Article/CJFDTOTAL-JXYE200204007.htm [15] 刘佳宁, 姜茂发, 李昱彤, 等.针铁矿法分离铬铁矿硫酸浸出液中Cr3+与Fe3+[J].过程工程学报, 2015, 15(2):242-246. http://www.jproeng.com/EN/abstract/abstract1200.shtml [16] 魏峰, 狄蕊, 杜锋, 等.含铬污泥酸浸出液中铬离子和铁离子的分离[J].检验检疫学刊, 2016(4):14-16. http://www.cnki.com.cn/Article/CJFDTOTAL-XDSJ201604005.htm [17] 邬建辉, 阳伦庄, 湛菁, 等.铬铁合金中的铬、铁分离研究[J].湿法冶金, 2011, 30(1):51-56. http://www.cnki.com.cn/Article/CJFDTOTAL-SFYJ201101019.htm [18] 高占博. 铬铁矿硫酸浸出新工艺的实验研究[D]. 沈阳: 东北大学. http://d.g.wanfangdata.com.cn/Thesis_J0102671.aspx [19] 杨振宁, 陈志传, 高大明, 等.电镀污泥中铜镍回收方法及工艺的研究[J].环境污染与防治, 2008, 30(7):58-61. http://www.cnki.com.cn/Article/CJFDTOTAL-HJWR200807019.htm [20] 郭峰. 混合电镀污泥中铬资源化工艺研究[D]. 赣州: 江西理工大学, 2014. http://cdmd.cnki.com.cn/Article/CDMD-10407-1015576987.htm [21] SPEIGHT J G. Lange's Handbook of Chemistry.(6th Edition)[M]. New York: McGraw-Hill, 2005.
[22] 张波, 杨达朋, 赵青, 等.铬铁矿硫酸浸出液中Cr3+与Fe3+的分离[J].有色金属(冶炼部分), 2014(7):1-3. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=mete201407001&dbname=CJFD&dbcode=CJFQ [23] 袁文辉, 徐志峰.混合电镀污泥中铬铁的选择性分离工艺[J].有色金属(冶炼部分), 2016(9):55-58. http://www.cnki.com.cn/Article/CJFDTOTAL-METE201609015.htm -
期刊类型引用(5)
1. 贺山明,潘界昌,徐国钻,李文君,梁勇. 粗钨酸钠溶液亚铁盐沉淀法除铬、钒的热力学分析及实验验证. 化工进展. 2023(04): 2171-2179 .  百度学术
百度学术
2. 梁贺磊,王东兴,杨声海,刘志强,曹洪杨,饶帅,张魁芳. 废三元锂离子电池浸出液中磷酸盐沉淀法除铝热力学分析及应用. 有色金属(冶炼部分). 2020(12): 36-41+68 .  百度学术
百度学术
3. 赵世强,郭丰. 三价铬电沉积过程强化规律研究. 有色金属科学与工程. 2019(04): 39-44 .  本站查看
本站查看
4. 陈建龙,邓炳林,梁余威,刘伟仁,樊文星. 铁泥废酸浸出液中铬铁分离的研究. 广东化工. 2018(20): 193-195 .  百度学术
百度学术
5. 刘牡丹,刘勇,陈志强,吕昊子,周吉奎,马致远. 添加剂对铜镍电镀污泥中重金属矿化的影响. 有色金属科学与工程. 2018(06): 60-64 .  本站查看
本站查看
其他类型引用(4)



 下载:
下载: