Zeolite modified and de-fluorination performance in zinc sulfate solution
-
摘要: 采用硝酸镧溶液浸泡负载元素镧的方法对天然沸石进行改性,通过静态吸附法研究改性沸石脱除硫酸锌模拟溶液的脱氟性能,考察改性沸石用量、pH值、吸附时间、吸附温度4个因素对改性沸石除氟性能的影响;并利用EDX、BET等分析手段对沸石改性前后的成分、形貌及微观结构进行表征。结果表明:当改性沸石用量为7.5 g/L,吸附时间25 min,反应温度为40 ℃,pH=4时改性膨润土的除氟量最大,氟离子浓度由100 mg/L降至45.56 mg/L,符合工业生产中所规定的小于50 mg/L;多种分析手段表明,改性后镧元素有效负载在沸石表面,同时沸石的层间距和比表面积都有所增加,有利于提高沸石的除氟性能.Abstract: Zeolite was used as the adsorbent and modified with lanthanum nitrate. An experimental study of the treatment of fluoride in zinc sulfate solution by modified zeolite was carried out. The influences of addition amount of modified bentonite,adsorption time,temperature and pH on the amount removal of fluoride were introduced. Energy dispersive X-ray analysis(EDX)and specific surface area(BET)were used to characterize the adsorbent before and after modified. The results show that the concentration of fluorine can be dropped from 100 mg/L to 45.56 mg/L,which conform to the provisions in the industrial production of less than 50 mg/L,when modified bentonite being added in an amount of 7.5 g/L,adsorption time being 25 min,temperature of 40 ℃ and pH=4. Variety of analytical tools show that,the lanthanum element payload on the surface of zeolite after the modification,and the layer spacing of zeolite and specific surface area are increased at the same time. I improves the fluoride removal performance of zeolite greatly.
-
Keywords:
- Lanthanum Nitrate /
- Modified Zeolite /
- De-fluorination /
- Static Adsorption
-
世界锌产量的80 %~85 %均由湿法工艺生产,即焙烧-浸出-净化-电积工艺。近些年由于社会的发展和进步,对资源的需求量也越来越高,但是矿产资源储备已经极度贫乏,大多数金属的富矿已经极为少见,各类矿石都已经面临“贫,细,杂”的窘境,锌矿石也不例外,直接导致锌精矿品位较低,杂质含量增加,特别是在冶炼过程中氟含量急剧增加,杂质元素氟的积累对后续净化及电解工艺产生严重危害[1]。电解过程中,氟离子属于腐蚀阴极的杂质离子,它能破坏阴极铝板表面的氧化铝膜,使析出的芯片与铝板表面形成锌铝固溶体,锌铝两种金属原子由于金属键作用而紧紧的结合在一起,发生锌铝粘结;另外,氟又是毒性高的物质,氟含量高会恶化工作环境及影响工人的健康。我国规定硫酸锌电解液中氟离子含量小于50 mg/L,因此,除去硫酸锌溶液中的氟离子是摆在我们面前的一项重大课题[2]。
目前,国内外处理硫酸锌溶液中氟的方法主要包括化学沉淀法、吸附法、混凝沉淀法、离子交换树脂法、反渗透法、液膜法、电渗析法等,较传统方法而言,吸附法具有适应条件广泛、操作方便、成本可控及吸附剂可再生使用等优点[3]。在此,对改性沸石这一吸附剂脱除硫酸锌溶液中氟的性能和机理进行研究。
1 实验
1.1 实验试剂与仪器
实验试剂:氟化钠(分析纯,西陇化工股份有限公司);硫酸锌(分析纯,成都市科龙化工试剂厂);天然沸石(分析纯,天津市科密欧化试剂有限公司);浓硫酸、浓硝酸(分析纯,株洲石英化玻有限公司);氢氧化钠(分析纯,湖南江虹试剂有限公司);柠檬酸三氨(分析纯,台山市粤侨试剂有限公司)等。
实验仪器:F-1型氟离子浓度计(南京科环分析仪器有限公司);雷磁232-01型参比电极(上海仪电科学仪器股份有限公司);85-2数显恒温磁力搅拌器(江苏中大仪器厂);BSA224S-CW电子天平(赛多利斯科学仪器有限公司);THZ-82数显水域恒温振荡器(常州国华电器有限公司);DZ-2BCⅡ真空干燥箱(上海贤德实验仪器有限公司);雷磁PHB-4便携式pH计(上海仪电科学仪器股份有限公司)等。
1.2 硫酸锌模拟溶液制备
硫酸锌模拟溶液主要成分见表 1.
表 1 硫酸锌模拟溶液主要成分(g·L-1)Table 1. Main components of zinc sulfate simulation solution(g·L-1)成分 Zn2+ H+ Fe3+ Fe2+ F- In 浓度 160.6 42.52 8.012 11.38 0.100 0.800 1.3 改性沸石的制备
将预先处理好的沸石,以固液质量比1:5加入到硝酸镧溶液中,放入恒温振荡器中,在35 ℃,150 r/min的条件下震荡浸渍4 h,然后从恒温振荡器取出静置半小时,用真空泵进行抽滤,随后将滤渣转移至100 mL烧杯中,放入真空干燥箱中在105 ℃下,干燥24 h,使其水分完全蒸干,然后从干燥箱中取出,用研钵将处理过后的块状沸石进行研磨,备用。
1.4 分析方法
氟离子选择电极法:溶液中的氟离子浓度用氟离子选择电极标准曲线法测定,氟离子选择电极为工作电极,饱和甘汞电极为参比电极,用直接电位法进行测定[4]。
2 结果与讨论
2.1 改性沸石的表征
2.1.1 X射线能谱分析
本次实验采用EDAX Genesis XM4 Neptune型能谱分析仪(EDX)进行表面观察与成分分析[5],利用EDX分析仪器自带软件进行谱线拟合。实验对沸石改性前后的主要成分进行检测。
天然沸石的元素含量如表 2所示,从表 2可以看出来,天然沸石主要以钠、铝、硅三种元素的化合物为主体物质,SiO2、Al2O3、Na2O的含量占据主体,构成沸石的框架。
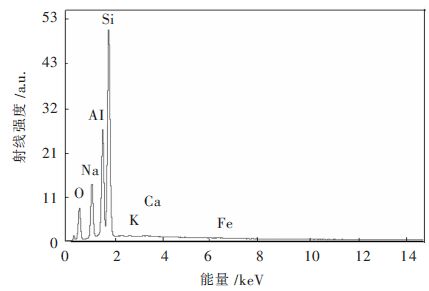
表 2 天然沸石元素含量表/%Table 2. Element content of natural zeolite/%物质 质量分数 摩尔分数 Na2O 13.49 14.77 Al2O3 26.11 17.38 SiO2 59.45 67.17 K2O 0.34 0.25 CaO 0.21 0.26 Fe2O3 0.39 0.17 图 1为天然沸石X射线能谱图,从图 1中可以得到,氧、钠、铝、硅元素的特征峰均较强,但是由于角度不同每种元素的峰的强度也有所不同,可能是由于这些元素在物质表面分布不均匀造成的。
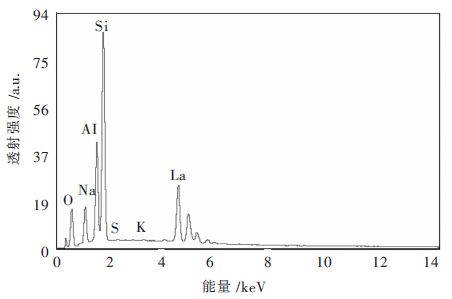
改性沸石元素含量如表 3所示,从表 3中可以看出来,改性沸石和天然沸石相比其构成物质主体的元素未发生改变,见图 2,但是相比于未改性的沸石,SiO2、Al2O3、Na2O三种物质的含量均不同程度减少,根据EDX检测原理可知,其探测深度较浅,由于改性物质的附着,导致其表面的部分结构物质被覆盖,在这里无法被检测到。在改性沸石的检测中发现含有大量的镧离子,这说明改性后镧离子以氧化物的形式存在于沸石中;在改性后CaO、Fe2O3未检测出来,而镧元素特征峰存在的位置正好是钙元素和铁元素特征峰所在的位置,由于改性过程中镧离子的大量增加,使得能量在4 keV~8 keV之间镧元素的特征峰将钙元素、铁元素的特征峰遮盖,因此无法反映出来[6-7]。
表 3 改性沸石元素含量表/%Table 3. Element content of modified zeolite/%物质 质量分数 摩尔分数 Na2O 7.48 10.69 Al2O3 19.71 17.12 SiO2 43.24 63.72 K2O 0.21 0.20 La2O3 28.99 7.88 通过天然沸石和改性后沸石前后能谱图以及元素含量表对比结果可以得知,本次实验在沸石上负载镧离子已经取得成功。
2.1.2 比表面积分析
比表面积是评价催化剂、吸附剂及其他多孔物质工业利用的重要指标之一。通过对沸石改性前后比表面积的测定,确定其吸附性能的变化程度。
从表 4所列沸石改性前后的BET检测结果可以看出,沸石本身的比表面积就比较大,通过查看资料可知,沸石是一种天然的多孔物质,具有很多细孔和微孔,可以为物质吸附创造很好的附着位置,提供较大的吸附空间[8]。但是,沸石通过改性之后,每克比表面积减少将近2 m2,这是由于改性过后,镧离子负载在沸石表面,其具有造孔作用,使得沸石表面较多微孔之间的孔壁消失,使微孔连接在一起产生介孔[9],与此同时,由于附着物在沸石表面,堵塞沸石表面的部分孔洞,使得沸石表面相比之前较为光滑,最后导致沸石改性后平均孔径增大,孔容增大,进而导致沸石的比表面积较天然沸石减小[10]。但由于改性后,沸石表面的电荷更接近于正电荷,对带负电荷的氟离子吸附能力增强;且镧离子自身的造孔作用,小孔和微孔,与大孔相比,微孔与小孔两两之间孔壁距离较近,孔壁产生的van der Waals势重叠,对物质分子的吸附作用力比中孔和大孔大,有利于其对物质的吸附[11]。因此,改性沸石与未改性沸石相比,吸附能力增强。
表 4 沸石改性前后的BET检测结果/(m2·g-1)Table 4. Zeolite BET test results before and after modification(m2·g-1)试样名称 天然沸石 改性沸石 比表面积 49.732 48.075 2.2 改性沸石脱除硫酸锌溶液中氟的实验研究
2.2.1 改性沸石加入量对吸附性能的影响
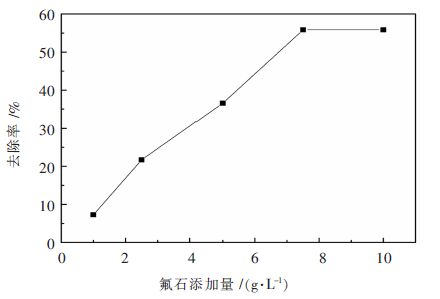
取不同量的改性沸石,共5组,温度40 ℃,pH=4.0,时间30 min,按照上述的实验步骤进行操作,得到其对氟离子的静态去除率A,结果见表 5和图 3。其中,去除率A=(氟离子剩余量-初始氟离子浓度)/初始氟离子浓度,初始氟离子浓度见表 1。
表 5 改性沸石加入量对吸附性能的影响Table 5. Influence of amount of modified zeolite on adsorption组号 沸石添加量/(g•L-1) 氟离子剩余量/(mg•L-1) 去除率/% 1 1 92.72 7.28 2 2.5 78.32 21.68 3 5 63.41 36.59 4 7.5 44.13 55.87 5 10 44.13 55.87 由表 5和图 3可以看出,改性沸石的在加入量较少时,其对氟离子的去除率小于10 %,随着改性沸石加入量的增加,改性沸石对氟离子的去除率急剧上升,当改性沸石的添加量到达7.5 g/L时,氟离子去除率随着改性沸石加入量的增加不再发生变化,去除率保持在55.87 %。由此可以看出,改性沸石去除硫酸锌模拟溶液中氟离子的最佳加入量为7.5 g/L。同时,当改性沸石加入量达到7.5 g/L时,硫酸锌模拟溶液中氟离子剩余量为44.13 mg/L,达到国家标准。
在改性沸石吸附过程中,加入量从1 g/L开始,变化到7.5 g/L,其吸附效果随着改性沸石加入量的增加而提高。这是由于改性沸石具有一定的表面积和大量的空隙,可以在硫酸锌模拟溶液中为氟离子提供一个类似于“床”的结构[12],氟离子在溶液中运动的过程中,受到此结构的阻碍而停留在改性沸石上;同时,氟离子为阴离子,改性沸石表面和空隙内部存在带正电荷的阳离子,正好由于静电吸附,正负离子相互结合,使得硫酸锌模拟溶液中氟离子被保留在改性沸石上。随着加入量的增加,其表面积和空隙也会随着变大,因此为氟离子的附着提供更多的“床位(活性位点)”[13],产生图 3在7.5 g/L之前氟离子去除率随着改性沸石加入量的增加而变大的现象。如图 3所示.
但是在7.5 g/L之后,改性沸石加入量不再影响其对氟离子的去除效果,这是由于,对于此时的溶液,虽然改性沸石的表面积和空隙进一步加大,但是其存在于硫酸锌模拟溶液体系中,溶液本身也存在大量的阳离子,在改性沸石吸附过程中,为了保证溶液中的电位平衡,阴阳离子在数量上保持稳定,溶液中的阳离子和改性沸石中的阳离子之间对氟离子的“争夺”出现一个平衡状态[14],所以出现图 3中在7.5 g/L~10 g/L之间氟离子去除率与改性沸石加入量的关系曲线和x轴平行的一段,因此改性沸石的最优加入量确定在7.5 g/L。
2.2.2 吸附时间对改性沸石吸附性能的影响
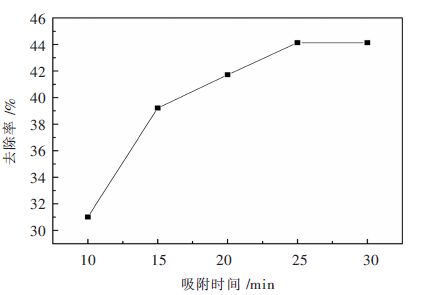
改性沸石加入量7.5 g/L,温度40 ℃,pH=4.0,按照上述的实验步骤进行操作,在不同的时间点对溶液中的氟离子浓度进行测量,结果见表 6和图 4。
表 6 吸附时间对吸附性能的影响Table 6. Influence of adsorption time on adsorption吸附时间/min 氟离子剩余量/(mg•L-1) 去除率/% 10 69.00 31.00 15 60.79 39.21 20 58.29 41.71 25 55.87 44.13 30 55.87 44.13 通过表 6和图 4可以看出,在吸附时间为10 min的情况下,改性沸石除氟率处在30 %以上,除氟效率相对不错。随着时间增加,改性沸石对氟离子的吸收能力随之提高;在10~15 min之间,其对氟离子的去除能力变化较大,当改性沸石的吸附时间达到25 min时,其对氟离子的吸附能力不再发生改变,稳定在45 %左右,吸附过程达到平衡状态。
从改性沸石在硫酸锌模拟溶液中存在开始,其吸附作用就得到展现。在10 min时,除氟率已经到达30 %以上,由图 5可以看出,在10~15 min的吸附过程氟离子的去除率增长较快,这是由于在10 min以前,硫酸锌模拟溶液和改性沸石刚刚接触,固-液表面形成一个过渡层,对氟离子通过溶液到达改性沸石表面有一定的阻碍作用[15];随着时间的推移,固-液之间存在的这个过渡层逐渐被打破,使得氟离子可以顺利得通过溶液和改性沸石接触,从而加快对氟离子的吸附。从15~25 min之间,去除率增长有所降低,这是因为在吸附过程中,硫酸锌模拟溶液中的氟离子逐渐减少,没有外加氟离子的情况下,吸附速率逐渐放缓;在25 min以后,改性沸石吸附氟离子的能力不随吸附时间的增加发生变化,这可能是因为它们表面吸附空位被占据和空位扩散速度的降低,使得吸附变得缓慢并且逐渐达到平衡[16]。
2.2.3 吸附温度对改性沸石吸附性能的影响
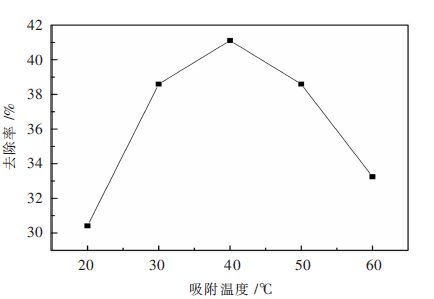
改性沸石加入量7.5 g/L,pH=4.0,吸附时间25 min,按照上述的实验步骤进行操作,分别进行5组不同吸附温度下的吸附实验,结果见表 7和图 5。
表 7 吸附温度对吸附性能的影响Table 7. Influence of temperature on adsorption组号 吸附温度/℃ 氟离子剩余量/(mg•L-1) 去除率/% 1 20 69.00 31.00 2 30 60.79 39.21 3 40 58.29 41.71 4 50 55.87 44.13 5 60 55.87 44.13 结合表 7和图 5,可以明显看出,吸附温度对整个吸附过程影响较大,在20 ℃的情况下,改性沸石对氟离子的去除率可以达到30 %,在20~30 ℃之间出现陡增现象,当到达40 ℃时改性沸石对氟离子的吸附能力最大,之后改性沸石对氟离子的吸附性能随着温度的进一步上升,下降极其明显。因此可得,当吸附温度为40 ℃时,改性沸石的吸附能力最强。
在上述吸附过程中,在20 ℃到40 ℃之间,随着温度增加,溶液体系中的氟离子热运动加剧,从而增加氟离子与改性沸石的接触机会;但是在40 ℃以上时,氟离子的去除率却与改性沸石的吸附温度出现负相关现象,这是由于在40 ℃以上,整个吸附过程的∆G<0,成为一个自发的反应,并且在离子被吸附后,其混乱度也会随之降低(∆S<0) ,由热力学公式∆G=∆H-T∆S可知,∆H必定小于零,故在40 ℃以上是一个放热反应,根据热力学平衡移动原理可以得出,升高温度对整个吸附过程不利[17]。因此,改性沸石吸附氟离子的温度应该保持在40 ℃为宜。
2.2.4 pH对改性沸石吸附性能的影响
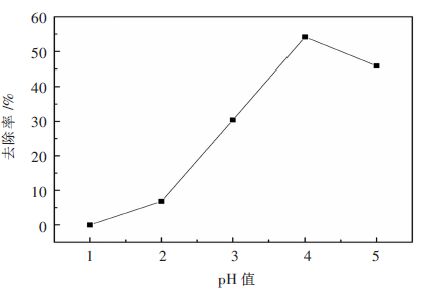
改性沸石加入量7.5 g/L,温度为40 ℃,吸附时间25 min,按照上述的实验步骤进行操作,分别进行5组不同pH值下的吸附实验,结果见表 8和图 6。
表 8 pH对吸附性能的影响Table 8. Influence of solution pH on adsorption组号 pH 氟离子剩余量/(mg•L-1) 去除率/% 1 1 100 0 2 2 93.21 6.79 3 3 69.59 30.41 4 4 45.84 54.16 5 5 54.17 45.83 由表 8和图 6可以得出,当pH为1时改性沸石几乎全部溶解,对氟离子的去除不起作用;当pH为2时改性沸石未发生溶解现象,其对氟离子的去除率较低,低于10 %;但是随着硫酸锌模拟溶液的pH不断变大,改性沸石对氟离子的吸附能力显著提高,除氟效率可达到50 %以上,不过当pH超过4时,改性沸石的除氟性能出现下降趋势,由图 6可以看出,硫酸锌模拟溶液中的pH为4时吸附效果最好。
在pH=1时,改性沸石出现溶解现象,这是由于整个硫酸锌模拟溶液体系中存在有大量的氟离子,在强酸性条件下会产生大量的HF,这使得原来沸石的铝硅酸盐晶体结构被“瓦解”,使得改性沸石出现溶解[18];当1<pH<4时,改性沸石对氟离子的去除率与pH成正相关的关系,这是由于在高酸度环境下,沸石表面发生质子化作用,吸附剂表面带有较多的正电荷,有利于对氟离子的吸附作用,但是在pH=1~2相比于pH=2~4的变化曲线斜率较小,这是由于在pH=1~2的情况下也形成部分HF,使溶液中的有效氟离子浓度降低,不利于氟离子的吸附;当pH超过4之后,改性沸石对氟离子的吸附能力随着pH的增加出现降低的现象,这是因为在相对较高的pH条件下,整个溶液体系中的OH-也随之增多,OH-作为阴离子也参与整个吸附过程,与氟离子产生竞争的关系,由于OH-的亲和势较强,占据氟离子在改性沸石上的位置,使得氟离子的去除率降低[19]。
3 结论
1)通过采用能量散射X射线荧光法(EDX)、比表面积分析(BET)两种检测方法检测得知,改性后沸石的基本成分未发生改变,保持其原有的状态,证实改性沸石在改性过程中改性物质均负载在沸石的表面,且分布较为均匀。
2)通过探究改性沸石加入量、吸附时间、吸附温度及pH对改性沸石吸附性能的影响,最终确定硝酸镧改性沸石除氟的最佳条件为:改性沸石加入量为7.5 g/L,吸附时间控制在25 min,吸附温度保持在40 ℃,溶液pH=4。
-
表 1 硫酸锌模拟溶液主要成分(g·L-1)
Table 1 Main components of zinc sulfate simulation solution(g·L-1)
成分 Zn2+ H+ Fe3+ Fe2+ F- In 浓度 160.6 42.52 8.012 11.38 0.100 0.800 表 2 天然沸石元素含量表/%
Table 2 Element content of natural zeolite/%
物质 质量分数 摩尔分数 Na2O 13.49 14.77 Al2O3 26.11 17.38 SiO2 59.45 67.17 K2O 0.34 0.25 CaO 0.21 0.26 Fe2O3 0.39 0.17 表 3 改性沸石元素含量表/%
Table 3 Element content of modified zeolite/%
物质 质量分数 摩尔分数 Na2O 7.48 10.69 Al2O3 19.71 17.12 SiO2 43.24 63.72 K2O 0.21 0.20 La2O3 28.99 7.88 表 4 沸石改性前后的BET检测结果/(m2·g-1)
Table 4 Zeolite BET test results before and after modification(m2·g-1)
试样名称 天然沸石 改性沸石 比表面积 49.732 48.075 表 5 改性沸石加入量对吸附性能的影响
Table 5 Influence of amount of modified zeolite on adsorption
组号 沸石添加量/(g•L-1) 氟离子剩余量/(mg•L-1) 去除率/% 1 1 92.72 7.28 2 2.5 78.32 21.68 3 5 63.41 36.59 4 7.5 44.13 55.87 5 10 44.13 55.87 表 6 吸附时间对吸附性能的影响
Table 6 Influence of adsorption time on adsorption
吸附时间/min 氟离子剩余量/(mg•L-1) 去除率/% 10 69.00 31.00 15 60.79 39.21 20 58.29 41.71 25 55.87 44.13 30 55.87 44.13 表 7 吸附温度对吸附性能的影响
Table 7 Influence of temperature on adsorption
组号 吸附温度/℃ 氟离子剩余量/(mg•L-1) 去除率/% 1 20 69.00 31.00 2 30 60.79 39.21 3 40 58.29 41.71 4 50 55.87 44.13 5 60 55.87 44.13 表 8 pH对吸附性能的影响
Table 8 Influence of solution pH on adsorption
组号 pH 氟离子剩余量/(mg•L-1) 去除率/% 1 1 100 0 2 2 93.21 6.79 3 3 69.59 30.41 4 4 45.84 54.16 5 5 54.17 45.83 -
[1] 郭天立,高良宾.硫酸锌溶液净化技术的现状与展望[J]. 有色矿冶, 2006, 22(1): 26-28. http://www.cnki.com.cn/Article/CJFDTOTAL-YSKY200601008.htm [2] 罗永光, 张利波, 彭金辉. 氧化锌烟尘湿法冶炼过程除氟现状与发展趋势[J].中国有色冶金, 2013, 42(4): 39-43. http://www.cnki.com.cn/Article/CJFDTOTAL-YSYL201304016.htm [3] 严刚. 镁型活化沸石除氟性能研究[J]. 青海大学学报(自然科学版), 2005(3): 9-11. http://www.cnki.com.cn/Article/CJFDTOTAL-QHXZ200503003.htm [4] EISHI Y, ATSUSHI S, MASATOSHI E. Removal of boron from wastewater by the hydroxyapatite formation reaction using acceleration effect of ammonia.[J]. Journal of Hazardous Materials, 2012, 237-238: http://cn.bing.com/academic/profile?id=1968807625&encoded=0&v=paper_preview&mkt=zh-cn
[5] 郑红, 戚洪彬, 梁树平. 大学化学实验[M]. 北京: 地质出版社, 2005. [6] 毕超. 镁、铁盐或其氧化物改性沸石水的除氟试验研究[D]. 济南: 山东建筑大学, 2010. [7] TANG Y L, GUAN X H, SU T Z, GAO N Y, WANG J M. Fluoride adsorption onto activated alumina: Modeling the effects of pH and some competing ions[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2009, 337(1):33-38 http://www.baidu.com/link?url=8p_C1gjoUfzlT5L7NsSjH5zmF8KyRgIMi0yg9R6Aei5YfGePTvgPWiOLMY9EGi1UQzHe_dF4JP8hfCcO3TLBTqFn6cdB33kYVB2h08mTiytZ0JV-NC2vTWH_BsyT_kPei-nl2AtalQV6B7C7ef84De-GWuE5-49j43qE-G_7HNW-ZshdRZCvwiUVFOwkeueDcpUifwDINHMZIvyN2iFY5GaEw2cVTt2fBQJ2dwrc044vGAO-heEt7airNDxKE3qN5Xxa9yeQUb2ZPxF67hXsQxCdrDn_ivQX-yMnnrlBb-pZ_VrmKXhmmOauPp507WaLBP0WzauLO1k-wnRLpunkFgstw8VlaplXYuzoyyj_BxRIn3dlHrL71D-KHvzfmY0s&wd=&eqid=b9f51b0700023015000000055861cc0e
[8] 席承菊. 沸石的改性及其除氟性能研究[D]. 哈尔滨: 东北林业大学, 2009. [9] 苏银银. 阳离子改性剂负载沸石对阴离子染料和小分子有机物的吸附研究[D].郑州: 郑州大学, 2014. [10] BHATNAGAR A, KUMAR E, SILLANPAA M. Nitrate removal from water by nano-alumina: Characterization and sorption studies[J]. Chemical Engineering Journal 2010, 163(3):317-323 doi: 10.1016/j.cej.2010.08.008
[11] DAS D P, DAS J, PARIDA K. Physicochemical characterization and adsorption behavior of calcined Zn/Al hydrotalcite-like compound (HTlc) towards removal of fluoride from aqueous solution[J]. Journal of Colloid and Interface Science, 2003, 261(2):213-220. doi: 10.1016/S0021-9797(03)00082-1
[12] MANDAL S, MAYADEVI S. Adsorption of fluoride ions by Zn-Al layered double hydroxides[J]. Applied Clay Science, 2008, 40:54-62. doi: 10.1016/j.clay.2007.07.004
[13] 刘泉利, 林海, 董颖博. 沸石颗粒表面电位调控、结构表征及应用[J]. 功能材料, 2013, 17(44): 2528-2532. http://www.cnki.com.cn/Article/CJFDTOTAL-GNCL201317022.htm [14] SOLANGI I B, MEMON S, BHANGER M I. Removal of fluoride from aqueous environment by modified Amberlite resin[J]. Journal of Hazardous Materials, 2009, 171:1-3. doi: 10.1016/j.jhazmat.2009.05.103
[15] SUJANA M G, SOMA G, VASUMATHI N, et al. Studies on fluoride adsorption capacities of amorphous Fe/Al mixed hydroxides from aqueous solutions[J]. Journal of Fluorine Chemistry, 2009, 130(8):749-754. doi: 10.1016/j.jfluchem.2009.06.005
[16] BISWAS K, SAHA S K, GHOSH U C. Adsorption of fluoride from aqueous solution by a synthetic iron(III)-aluminum(III) mixed oxide[J]. Industrial & Engineering Chemistry Research, 2007, 46(16):5346-5356.
[17] 尹玲玲, 谢雄华. 处理后沸石和改性D401树脂除氟研究[J]. 山西建筑, 2006, 32(l): 196-197 http://www.cnki.com.cn/Article/CJFDTOTAL-JZSX200601126.htm [18] 胡丽娟, 周琪. 活化沸石的饮用水除氟工艺研究[J]. 净水技术, 2005, 24(3): 15-18 http://www.cnki.com.cn/Article/CJFDTOTAL-ZSJS200503004.htm [19] 席承菊. 沸石的改性及其除氟性能研究[D]. 哈尔滨: 东北林业大学, 2009



 下载:
下载: