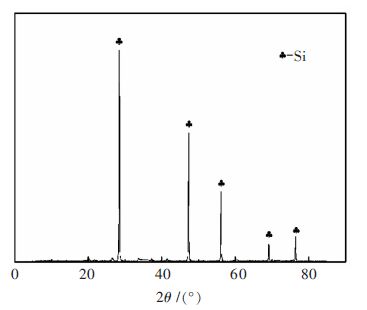
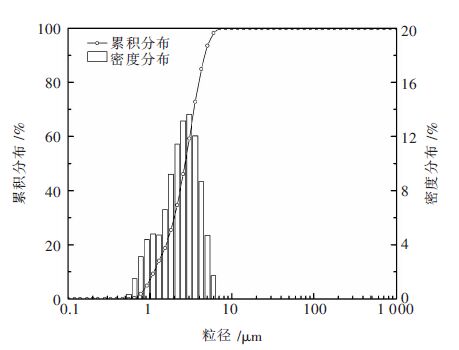
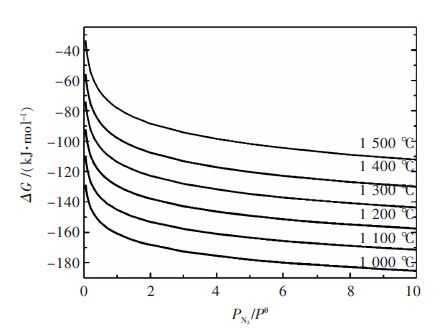
Thermodynamic analysis and kinetics mechanism for direct nitridation reaction
-
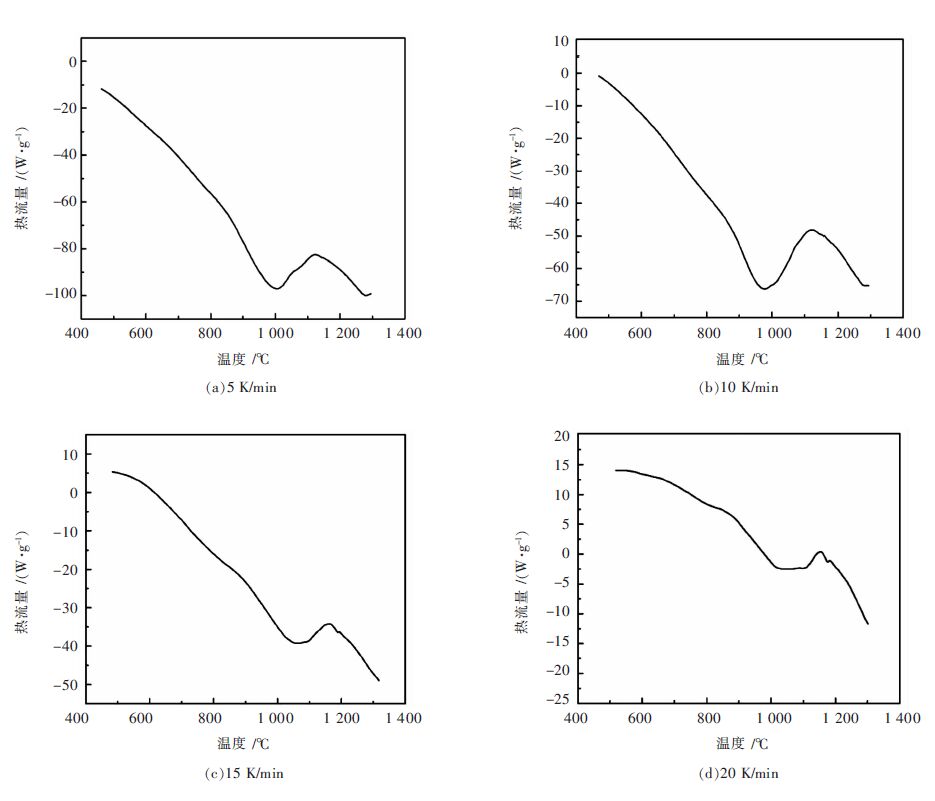
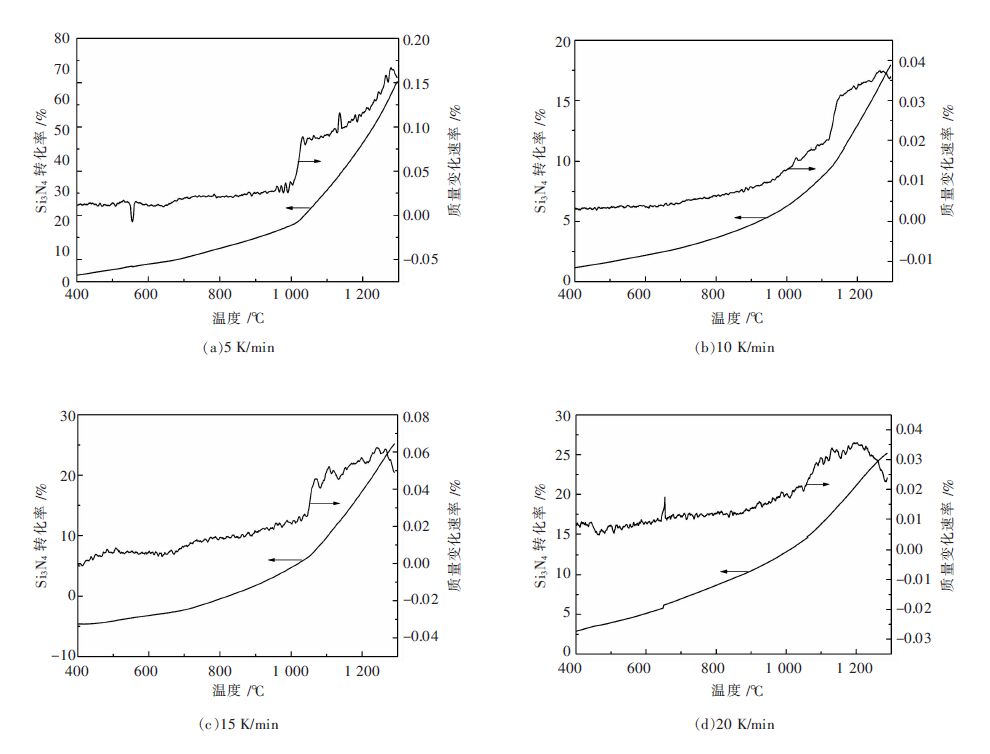
摘要: 用TG-DSC热重同步热实验分析的方法,研究不同升温速率下,硅粉氮化机理及化学反应动力学.发现温度在1 000~1 300 ℃时:差示扫描量热曲线各出现一个吸热、一个放热峰,说明氮化机理已发生改变.在1 000~1 100 ℃温度范围内,氮化硅转化率显著增加,即温度是影响其转化率的主要因素.实验表明:氮化反应的限制性环节由反应开始阶段的界面化学反应控制和之后的界面化学反应与内扩散混合控制组成;通过动力学计算得到表观活化能E=404.5 kJ/mol,频率因子A=9.57×1015 m/s,反应级数n=0.95,最终得到反应的速率方程的数学表达式.Abstract: The mechanism and chemical kinetics of nitridation reaction at different heating rates was investigated by thermogravimetry. It is found that there is an exothermic peak and an endothermic peak in the differential scanning calorimetry curve at 1 000~1 300 ℃ centigrade, which indicates that the mechanism has changed. The conversion rate of silicon nitride is significantly increased within 1 000 to 1 100 ℃, and it indicates temperature is the dominant factor affecting the conversion rate. Experiments results show that the restrictive step of nitridation reaction is composed of chemical reaction in early stage of reaction and the mixed-control of chemical reaction and intraparticle diffusion in the subsequent reaction; the apparent activation energy is kinetically calculated to be 404.5 kJ/mol, the frequency factor A=9.57×1015 min/s, the reaction order n=0.95, and mathematical expression of reaction rate equation is finally obtained.
-
Keywords:
- silicon nitride /
- thermogravimetric analysis /
- thermodynamics /
- kinetic modeling /
- synthesis
-
以回采时地压的管理方法为依据,地下矿山所沿用的采矿方法可分为3类,即空场采矿法、崩落采矿法以及充填采矿法[1].这3类采矿法有各自的使用特点,在我国的采矿事业中取得了长足的发展.随着国家对环保要求的不断提高,充填采矿法得到了快速发展,尤其是胶结充填采矿法的应用,很好地解决了矿山企业尾矿库库容问题,减少了矿山企业对尾矿库的运行及安全管理的费用[2].充填采矿法能够很好的适用于各种复杂的矿山地质条件,在深部开采中尤为明显,适合开采贵重金属及附近存在重要工程设施的地下矿山[3].
近年来,胶结充填采矿法在我国矿山行业中得到了广泛应用,在相应的理论研究领域也取得了重要成果.在众多研究中,充填材料是使得这一采矿技术能够得到广泛应用的关键.国内很多专家学者就充填材料方面做了大量研究,如采用不同配比的充填体力学性能研究,分级尾砂充填工艺的研究,全尾砂胶结充填采矿技术的应用等[4-9].但对于充填材料中所使用的胶结剂、其力学性能以及破坏机理等研究还不够完善[10-15].目前,矿山正常生产中使用最多的是以水泥作为胶凝材料,缺点就是凝结时间长,在胶结尾砂过程中,将发生水化反应释放出大量热量.因此,提出新型固化剂替代水泥作为尾砂充填体的胶结材料,研究不同配比下2种分级尾砂胶结充填体充当采场顶板的力学性能及稳定性,解决采场回采工作面温度过高等问题,具有重要的工程应用价值[16-20].
文中以某铜矿北矿带为工程背景,采用下向进路式分层胶结充填采矿法,将阶段矿块划分为4个分层进行回采,每分层回采后,先采用1:4的水泥和分级尾砂浆体充填至分层一半的高度,剩余部分采用1:8的浆体充填至上部分层的底部并接顶.为了解决采场温度过高的问题,现采用1:6和1:12两种配比的新型固化剂胶结充填体分别替代1:4和1:8水泥胶结充填体,研究在下层回采分条采空的情况下,上层分级尾砂胶结充填体作为顶板的变形情况,进而分析采用新型固化剂胶结的分级尾砂胶结充填顶板的稳定性,以及对第4分层的开采是否能够满足安全作业的要求.
1 现场试验
1.1 测试方案
采用下向进路式分层胶结充填采矿法的矿山,当第4分层的矿块回采时,第1、2分层充填体直接作用在第3分层充填体上,此时第3分层充填体承受了垂直方向上3个分层充填体的应力与变形.因此,第3分层作为最关键的分层,其稳定性直接影响到第4分层的安全回采工作能否顺利进行,下向分层充填采矿法结构体如图 1所示.
试验选择某铜矿北矿带-310 m中段E4盘区试验分层第3试验分层的5回采分条和5’回采分条作为试验分条.为了测得胶结充填体充当采场顶板的变形量的试验数据,根据混凝土应变计需埋设要求,按照试验方案以垂直和水平正交3个方向布置在试验采场的2个回采分条中.竖筋布置在试验采场回采结束之后,在选定的竖筋上安装振弦式钢筋测力计,然后再布置竖筋,待采场内钢筋应力计及竖筋安装完毕后,将试验采场用胶结分级尾砂一次充填.直至第4分层回采后,揭露相应埋设仪器下方充填体部位时,通过监测获取相应的数据并进行处理,分析该分层暴露后整个充填顶板的变形特征和受力情况,以及固化剂胶结充填材料作为采场顶板的稳定性.
1.2 仪器安装
采场充填前,在沿着进路和垂直进路的3个方向上,选择合适的位置布置3根铁丝网,并将3个应变计以互相垂直的方式固定在铁丝网上并绑扎牢固.钢筋混凝土测力计需和竖筋连接在一起使用,将3个钢筋测力计按照上、中、下均匀地以螺纹连接的方式安装在每一根竖筋上.为了保证仪器正常工作的可靠性,试验仪器安装完毕后,分别用读数仪进行检查,避免仪器安装过程中出现损坏以便及时更换,并记录下应变计和测力计的初始读数.最后,将应变计和测力计的电缆拉至充填挡墙以外.
1.3 测点布置
针对最为关键的第3分层充填体进行现场应力监测,分析第4分层矿体开挖前后第3分层充填体顶板的整体稳定性及受力变形情况.选择第3分层中5和5’两个分条作为测试对象,埋设混凝土应变计和钢筋应力计,监测其对应的下层矿体开采前后顶板的应力和位移值.各监测点布置如图 2、图 3所示.第3分层5’分条钢筋应力计和应变计的布点设置及对应编号如表 1、表 2所列.
表 1 第3分层5’分条应变计编号与布点编号对照Table 1. Third hierarchical 5'points of strain gage ID number and distribution table布点位置 测点方向 应变计编号 上部 沿进路方向 35’B01 垂直进路方向 35’B02 垂直作业面方向 35’B03 中部 沿进路方向 35’B04 垂直进路方向 35’B05 垂直作业面方向 35’B06 下部 沿进路方向 35’B07 垂直进路方向 2FB08 垂直作业面方向 2FB09 表 2 第3分层5’分条钢筋计编号与布点编号对照Table 2. Third hierarchical 5'points of reinforcement meter ID number and distribution table布点位置 钢筋计编号 1号 2号 3号 4号 5号 6号 上部 35’G01 35'G04 35'G07 35'G10 35'G13 35'G16 中部 35’G02 35'G05 35'G08 35'G11 35'G14 35'G17 下部 35’G03 35'G06 35'G09 35'G12 35'G15 35'G18 1.4 监测结果及分析
对于第3试验分层,由于部分监测仪器在充填过程中被损坏,没有监测到顶板的应力和位移值.因此,根据正常工作的5’分条混凝土应变计和钢筋应力计监测数据,监测结果如图 4、图 5所示.
2 数值计算
2.1 计算模型
在模拟试验中,结合现场竖筋应力监测结果,对模型中的竖筋施加相应的预应力,模拟竖筋的受力状态,分别采用新型固化剂胶结尾砂充填体和42.5级水泥胶结尾砂充填体作为第4回采分层的采场顶板.根据弹性力学理论,由于回采分条断面宽度远小于整个采场开采断面长度(两者之间尺寸之比为1:13),因此,可以通过建立二维的平面模型对其进行数值计算.根据矿山实际采用的矿块回采规格,以4 m×3.5 m (宽×高) 作为分条回采进路断面尺寸,依据圣维南原理,回采工作面附近的矿岩体受开采扰动影响较大,其影响区域大致在开采断面尺寸5倍以上的范围内.通过理论分析,最终确定用于计算的模型几何尺寸84 m×54 m (宽×高) 是合理的.
模拟试验的重点是充填体顶板的稳定性以及埋设的竖筋在充填体中的受力状态.为了提高计算的精确性,突出重点部位的变形特征及力学机理,分别采用网格基本单元长度为0.05 m和0.01 m对钢筋托盘和竖筋附近的网格进行加密处理,充填体和矿岩的网格基本单元长度为1 m,整个模型共有9 919个计算单元和8 765个节点组成.每排竖筋垂直于回采进路方向间隔2 m布置.分析过程中,为了对模型进行简化,减少计算的时间使得模拟方便,将上部岩层的重量通过施加垂直应力来实现.计算模型如图 6所示.
2.2 本构模型及边界条件
用于数值计算的弹塑性材料,可采用Mohr-Coulomb屈服准则,判断其受剪切破坏时的依据为:
$${f_s} = {\sigma _1} - {\sigma _3}{N_\varphi } + 2c\sqrt {{N_\varphi }} $$ 其中:
$${N_\varphi } = \frac{{1 + \sin \varphi }}{{1 - \sin \varphi }}$$ 其中,σ1为最大主应力;σ3为最小主应力,c为材料的黏聚力;φ为材料内摩擦角. fs为判断材料是否破坏的系数,当fs≥0时,材料处于塑性流动状态,认为材料已经被破坏;当fs≤0时,材料处于弹性变形阶段,认为材料仍具有完整的力学性能.
根据矿房的开采深度以及上覆围岩的容重,在模型的上边界向下施加0.03 MPa的垂直应力,模型的左右边界限制水平方向位移,模型下边界固定.
2.3 材料参数选择
根据矿山选用的竖筋规格,其力学参数如表 3所列.
表 3 钢筋力学参数Table 3. Steel reinforced mechanical parameters参数名称 直径
/mm长度
/m屈服点
/MPa抗拉强度
/MPa弹性模量
/MPa参数指标 16.0 2.4 335 490 200 通过室内试验得到的矿岩、围岩以及不同配比胶结充填体的力学参数如表 4所示.
表 4 岩体及充填介质力学参数Table 4. Mechanical parameters of rock mass and filling medium类型 度/(kN·m-3) 性模量/GPa 泊松比 度/MPa 结力/MPa /(°) 围岩 25.87 20.83 0.20 2.50 3.00 44 矿体 33.89 10.63 0.26 1.20 2.20 36 1:12固化剂充填体 19.50 0.45 0.29 0.40 0.50 17 1:6固化剂充填体 19.50 0.73 0.25 0.60 0.70 19 1:8水泥充填体 20.00 0.42 0.27 0.36 0.50 16 1:4水泥充填体 20.00 0.69 0.24 0.50 0.60 18 2.4 计算结果及分析
为了研究充填体顶板的稳定性,数值模拟分别以第3试验分层的固化剂胶结分级尾砂充填体和42.5级水泥胶结分级尾砂充填体作为第4回采分层的顶板,研究第3分层充填体加入竖筋和不加竖筋条件下,矿体回采后回采进路巷道的稳定性及顶板的应力与位移量.以试验采场为例,采用从中间对称的开挖方式,研究采用1:6与1:12新型固化剂充填体和1:4与1:8水泥胶结充填体,在布置竖筋与不布设竖筋条件下,下分层矿体回采后顶板的稳定性,并对整个采场充填工艺进行优化.由于篇幅所限仅列出1:6和1:12两种配比的新型固化剂胶结分级尾砂充填的计算结果.
1)应力分析.通过对采用1:6和1:12两种配比的新型固化剂胶结分级尾砂充填体充当下分层回采顶板的应力分析,得到了不同回采步骤下顶板充填体的应力图,如图 7所示.
2)位移分析.通过对采用1:6和1:12两种配比的新型固化剂胶结分级尾砂充填体充当下分层回采顶板的位移分析,得到了不同回采步骤下顶板充填体的位移图,如图 8所列.
3)塑性区分析.通过对采用1:6和1:12两种配比的新型固化剂胶结分级尾砂充填体充当下分层回采顶板的塑性区分析,得到了不同回采步骤下顶板充填体的塑性区分布图,如图 9所列.
通过对采用质量比为1:6和1:12的固化剂胶结充填体(加竖筋)的应力、位移及塑性区进行分析,其结果如表 5所列.
表 5 采用质量比为1:6和1:12固化剂胶结充填(加竖筋)Table 5. Using mass ratio of 1:6 and 1:12 curing agent cemented agent filling and vertical reinforcement开挖步骤 垂直方向应力/kPa 垂直方向位移/m 塑性区分布 1 200 0.004 5 无 2 500 0.008 0 无 3 500 0.008 0 侧帮充填矿柱有小块塑性区 4 500 0.010 0 侧帮充填矿柱有小块塑性区 5 600 0.010 0 侧帮充填矿柱有小块塑性区 6 600 0.010 0 侧帮充填矿柱有小块塑性区 1)采用质量比为1:6和1:12新型固化剂胶结分级尾砂分层充填.通过数值模拟,分别对6个开挖步骤下新型固化剂胶结分级尾砂充填体作为第4分层回采顶板的稳定性进行了数值计算,通过将钢筋应力计监测到的应力施加到相应的回采顶板的竖筋上,计算结果表明:通过垂直方向应力分析,得到在回采过程中顶板的垂直方向上应力变化不大,在第5、第6步回采过程中,顶板垂直方向上应力出现小幅增加,但仍在顶板抗拉强度承载的范围内,不至于出现失稳破坏;通过对其垂直方向位移分析,得到至开挖结束顶板的垂直方向上位移量极小,最大位移值为0.01 m,与现场上部分层监测的位移值接近;通过对各回采步骤的塑性区分析,各回采步骤结束后顶板都未出现塑性区,在第5、第6步回采过程中,侧帮充填体,出现很小的塑性区,对采场顶板的稳定性没有影响.
2)采用质量比为1:4和1:8水泥胶结分级尾砂分层充填.为了对比新型固化剂充填体充当采场顶板的效果,采用同样的方式,以现场监测的钢筋应力计读数施加到数值模型的竖筋上,计算结果表明:通过应力分析,由于2种材料的物理力学性质相近,因此其垂直方向上应力变化趋势与采用新型固化剂材料表现一致,计算得到的应力值稍大于新型固化剂材料;通过位移分析,其垂直方向位移计算结果同样与采用新型固化剂材料保持高度相似性;通过塑性区分析,其回采至第3步,顶板上方开始出现小块塑性区,在其后回采过程中,塑性区面积显著增大,并且侧帮充填体出现了多块塑性区部位,对顶板的稳定性有一定的影响.
3)分别采用质量比为1:6和1:12新型固化剂胶结分级尾砂一次充填.为了简化充填工艺,采用同一种配比的新型固化剂胶结分级尾砂一次性充填采空区,研究其作为第4分层回采顶板的稳定性,同样在充填体中布置竖筋,计算结果表明:采用1:12的新型固化剂胶结充填体一次充填时,当回采第1步时,顶板便出现了多块面积较大的塑性区,顶板的垂直方向位移量较大;采用灰砂比为1:6的新型固化剂胶结充填体一次充填时,顶板处没有塑性区出现,在上覆围岩中产生大面积塑性区,但垂直方向上的应力和位移明显大于采用灰砂比为1:6和1:12新型固化剂胶结分级尾砂分层胶结充填时的应力值和位移值.
4)未布置竖筋采用质量比为1:6和1:12新型固化剂胶结分级尾砂分层充填.为了研究竖筋在充填体中是否起到加固的作用,本次模拟采用不布置竖筋的新型固化剂胶结分级尾砂分层充填,计算结果表明:第1步回采结束后,上部充填体顶板出现几块小面积塑性区,垂直方向上位移值偏大;第2步回采结束后,在侧帮矿岩与顶部充填体接触的部位产生大面积塑性区,顶板垂直方向位移值超过0.012 m.
3 结论
1)通过第3分层的应变计和应力计监测数据结果表明,充填体暴露为顶板之前曲线较为平缓,其应力和应变值较小,变化不大.结合上两层应力应变监测结果,充填体暴露为顶板时,曲线斜率增大,之后一段时间内趋于稳定,推测第4分层回采后,第3分层充填体表现为相同的变化趋势.
2)采用加两排竖筋且质量比为1:6和1:12的新型固化剂胶结分级尾砂分层充填替代质量比为1:4和1:8的42.5级水泥胶结分级尾砂分层充填,顶板无塑性区出现且垂直方向位移量较小.分别采用质量比为1:6和1:12的新型固化剂胶结分级尾砂一次充填作为下层回采分层的顶板时,顶板暴露后,位移变形量增大,顶板出现多块大面积塑性区,呈失稳状态.因此,对于采用质量比为1:6和1:12的新型固化剂胶结分级尾砂分层充填空区是合理的,能满足采场回采时顶板安全控制的要求.
3)通过对竖筋在充填体中的应力分析,未布设竖筋的顶板处于失稳状态,其在新型固化剂材料中起到了加强顶板整体强度的作用,且力学效果明显.通过验证,直径为16 mm的竖筋能满足采场稳定性的要求.
-
表 1 硅粉的化学组成
Table 1 Chemical composition of silicon powder
成分 Si Al Ca Fe 含量/% ≥99.999 ≤0.001 ≤0.000 1 ≤0.000 1 表 2 高纯氮气的化学组成
Table 2 Chemical constituents of high-purity nitrogen
成分 N2 O2 H2O H2 总碳氢化合物 含量/% ≥99.999 ≤1.0×10-6 ≤1.0×10-6 ≤0.5×10-6 ≤1.0×10-6 表 3 不同升温速率下硅粉氮化反应的DSC 峰温数值
Table 3 DSC peak temperature values of nitriding reaction of silicon powder at different heating rates
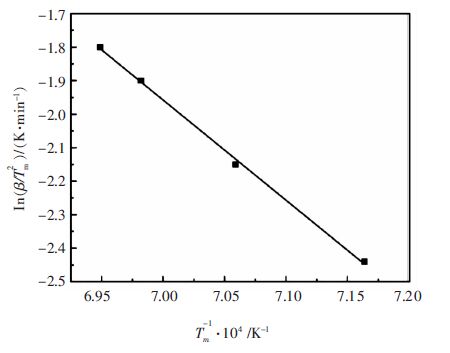
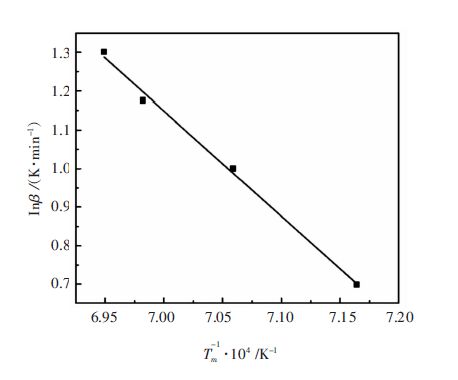
β/(K·min-1) Tm /K 5 1 395.94 10 1 416.64 15 1 432.16 20 1 439.06 -
[1] SHUKLA P P, LAWRENCE J. Fracture toughness modification by using a fibre laser treatment of a silicon nitride engineering ceramic[J]. Journal of Materials Science, 2010, 45(23): 6540-6541. doi: 10.1007/s10853-010-4743-6
[2] 鲁元,杨建锋,李京龙. 碳热还原-反应烧结法制备多孔氮化硅陶瓷[J]. 无机材料学报, 2013, 28(5): 469-473. http://www.baidu.com/link?url=GZ4-5b11H224VJz2TEhTN8QlI9bjSEyAbeRy4oPH38SuYaD-vUYRRpEsQEsxzQSx55mvAlgKHeBXMAPqQ2fIL64NVDEqjydIpR-EfMOwSOWP0aqh-jtj-fpQupfkROAHnUgHv8LF996e4G3HWJHPNKtUF9nMdGrV8xF5xydv3yeB-sKUfjsi1ZuYN8NsFE0FVoFPaTJqIjnhxJShvkmo5ooKOknWO33IHhTCaOyf5cshb1s-r76B0BtTRPiORf7jzvHrFV6IDgGCsb4X6ziXetybGa02rXlEZP1mUQVuTKKHWmbhzn06VMI9vlGZPwI0G1p3WxWMooBkvOdFzCxHeU88iLqUtR7Q_0bYF60lowHwO5t3aJsVh9f6dgvuDGtC20RBR8tRW6EaqAMBC1uHi7PhZNOeMYH_t_WG90P4WbYOklQEZ76xx3znopLFcLEBH0rg0uuwzjSkbZ4mm9VpGK&wd=&eqid=86998a3500032b1a00000005581019e0 [3] 李金富,李康,李拥军,等. 工艺参数对自蔓燃制备氮化硅粉体的影响[J]. 硅酸盐通报,2007, 26(2): 252-255. http://www.cnki.com.cn/Article/CJFDTOTAL-GSYT200702007.htm [4] 李亚伟,张忻,田海兵,等. 硅粉直接氮化反应合成氮化硅研究[J]. 硅酸盐通报, 2003, 22(1): 30-34. http://www.cnki.com.cn/Article/CJFDTOTAL-GSYT200301007.htm [5] 王勇,沃银花,姚奎鸿,等. 流态床CVD 法纳米氮化硅粉体的制备[J]. 无机材料学报, 2006, 21(1): 41-45. http://www.cnki.com.cn/Article/CJFDTOTAL-WGCL200601007.htm [6] 李虹,黄莉萍,蒋薪. 碳热还原法制备氮化硅粉体的反应过程分析[J]. 无机材料学报, 1996, 11(2): 241-246. http://www.cnki.com.cn/Article/CJFDTOTAL-WGCL602.007.htm [7] 万小涵,张广清,JOHN SHARP,等. 高氮分压对碳热还原/氮化法合成氮化硅的影响[J]. 有色金属工程,2015, 5(4): 9-12. http://www.cnki.com.cn/Article/CJFDTOTAL-YOUS201504003.htm [8] 马啸尘,尹洪峰,张军战,等. 以木屑为碳源制备氮化硅粉体的研究[J]. 耐火材料, 2015, 49(1): 31-35. http://www.cnki.com.cn/Article/CJFDTOTAL-LOCL201501009.htm [9] 古亚军,曹迎楠,李发亮,等. 铁纳米颗粒催化氮化硅粉[J]. 硅酸盐学报, 2014, 42(12): 1585-1589. http://www.cnki.com.cn/Article/CJFDTOTAL-GXYB201412019.htm [10] 李勇,朱晓燕,王佳平,等. 反应烧结氮化硅–碳化硅复合材料的氮化机理[J]. 硅酸盐学报,2001, 39(3): 447-451. http://www.cnki.com.cn/Article/CJFDTOTAL-GXYB201103014.htm [11] 高梅,李勇,秦海霞,等. 闪速燃烧合成氮化硅铁的氮化机理[J]. 硅酸盐学报, 2015, 43(3): 358-362. http://www.cnki.com.cn/Article/CJFDTOTAL-GXYB201503019.htm [12] 李绍芬. 反应工程[M]. 3版. 北京: 化学工业出版社, 2013. [13] 郭汉杰. 冶金物理化学[M]. 北京: 高等教育出版社, 2006. [14] 杨福明,王立,尹少武,等. 硅粉常压直接氮化过程的非催化气固反应模型[J]. 北京科技大学学报,2013, 35(6): 785-792. http://www.cnki.com.cn/Article/CJFDTOTAL-BJKD201306012.htm [15] KISSINGER H E. Reaction kinetics in differential thermal analysis[J]. Analytic Chemistry, 1957, 2(11): 1702-1704.
[16] 沈兴. 差热、热重分析与非等温固相反应动力学[M]. 北京: 冶金工业出版社, 1995. [17] STAVA V, SETAK J. Computer calculation of the mechanism and associated kinetic data using a non-isothermal integral method[J]. Journal of Thermal Analysis and Calorimetry, 1975, 8(3): 477-489. doi: 10.1007/BF01910127




 下载:
下载: