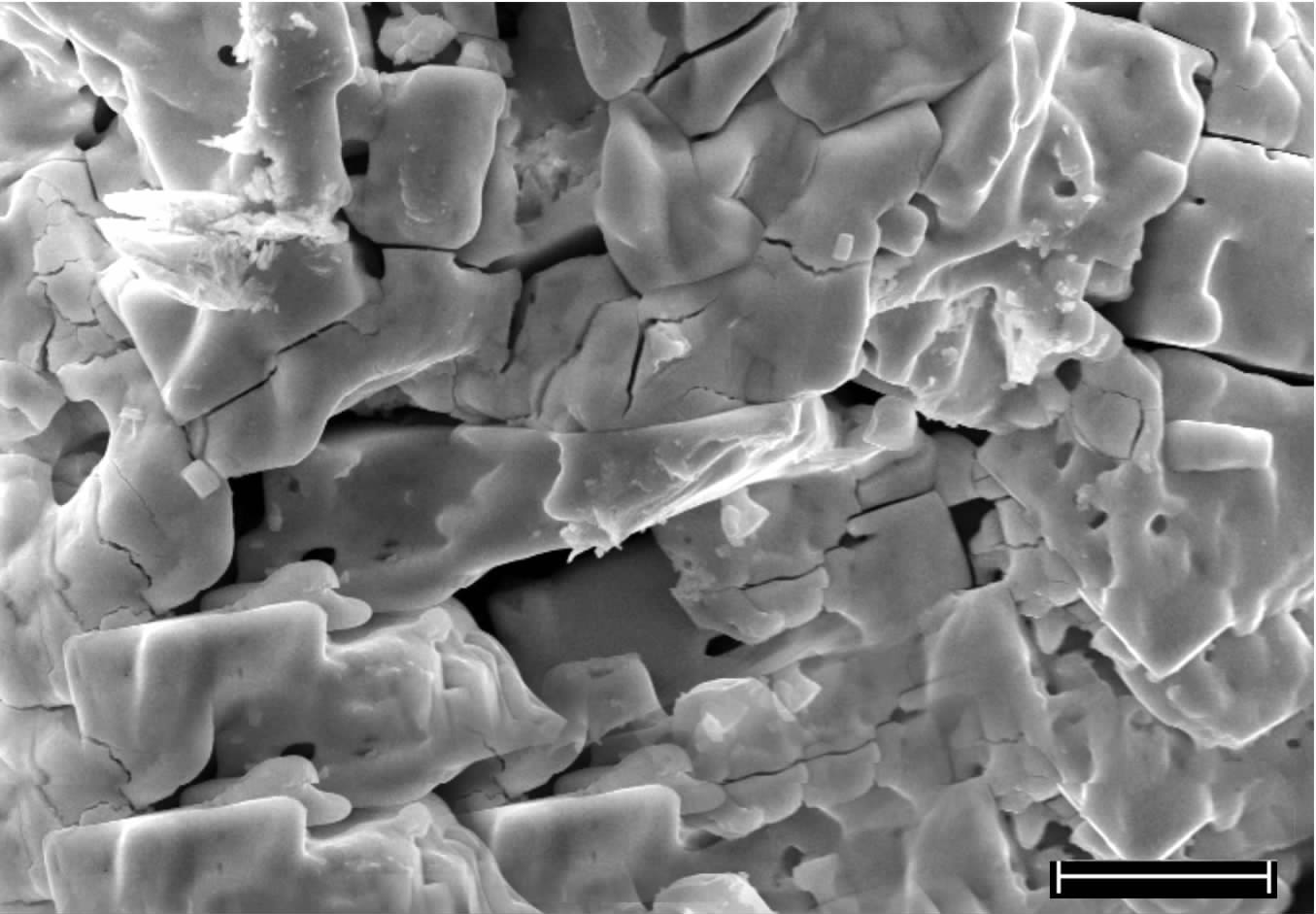
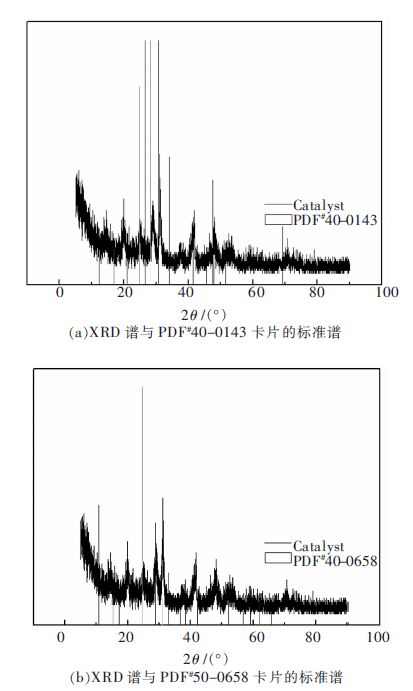
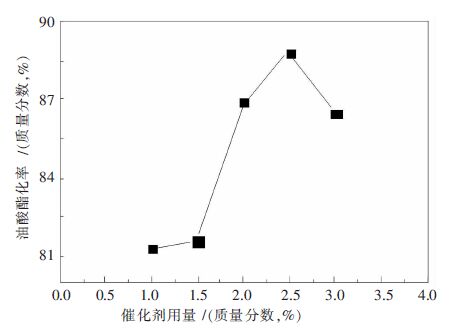
Catalytic activity of La-modified phosphotungstic heteropoly acid salt in synthesis of biodiesel from esterification of methanol and oleci acid
-
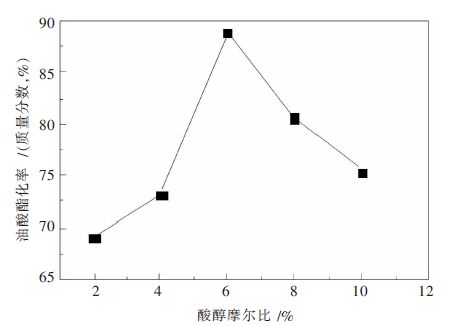
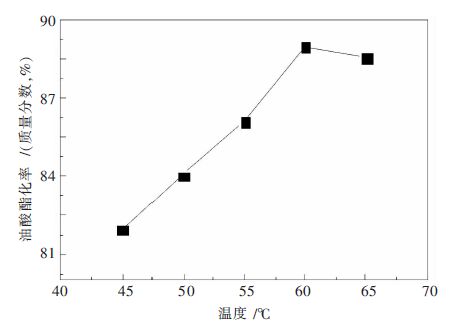
摘要: 以H 3PW 12O 40和La(NO 3) 3为原料,通过离子交换法制备一种稀土镧改性磷钨杂多酸盐催化剂.通过扫描电镜、红外光谱和X射线粉末衍射等表征方法,对合成的催化剂的物理及化学性能进行分析,进而通过以油酸和甲醇为反应物的酯化反应,对其催化活性进行研究.结果表明:稀土镧已经导入磷钨杂多酸的骨架结构,并与杂原子P形成配位键,有效提高磷钨杂多酸的比表面积和孔径;合成的催化剂具有完整的Keggin型结构;当反应温度为62 ℃,油酸与甲醇摩尔比为1∶6,反应时间为4.5 h,催化剂用量为反应物质量的2.5 %,油酸的转化率可达88.0 %.Abstract: La-modified phosphotungstic heteropoly acid salt catalyst was prepared by using H 3PW 12O 40 and La(NO 3) 3as raw materials through the ion exchange method. The physical and chemical properties of the obtained-catalyst were analyzed with the employment of scanning electron microscopy, infrared spectroscopy and X-ray powder diffraction. The catalytic activity of the obtained-catalyst was studied in the esterification reaction with the oleic acid and methanol as raw materials. The results show that the rare earth lanthanum has been introduced into the skeleton structure of phosphotungstic heteropoly acid and formed new coordination bond with the heteroatom P, which effectively improved the specific surface area and pore size of phosphorus tungsten heteropoly acid; La-modified phosphotungstic heteropoly acid salt keeps the Keggin structure of phosphotungstic heteropoly acid completely. A yield of oleic acid methyl ester 88.0 % is obtained when the reaction temperature is 62 °C, the molar ratio of methanol and oleic acid is 1:6, reaction time is 4.5 h, and the mass ratio of catalyst to the reactants (oleic acid and methanol) is 2.5 %.
-
Keywords:
- are earth /
- lanthanum /
- heteropoly acid salt /
- esterification reaction /
- biodiesel
-
钴酸锂锂离子电池是全球电池市场中占比较大的电池种类,从2000年到2004年,全球锂电池总产量从每年5亿块增加到每年7亿块,2010年全球年产量已达46亿块.在中国,仅2014年就生产了52.87亿块锂离子电池,占全球总产量的72 %[1-5].锂离子电池产量如此庞大,导致的另一个问题便是每年被废弃的电池数目也十分惊人[6-10].锂离子电池中含有Co、Cu、Li、Al、Fe等金属元素,如果随意丢弃,不仅会对环境以及人们健康造成危害,还会浪费宝贵的金属资源[11-15].因此废旧锂离子电池中金属的回收具有重要的意义.
现阶段主要有中和法、碱浸法、有机溶剂萃取法[16-18]等方法来回收锂离子电池废料中的Al, 但是采用中和法效果不理想,萃取法中的有机溶剂又对人体有害.萃取剂成本相对于碱浸法成本较高,碱浸法所需的氢氧化钠成本相对较低,故文中重点研究在碱浸除铝工艺,通过调节碱浸除铝工艺参数,提高碱浸工艺中铝浸出率,为后续有价金属的回收提供方便,碱浸后液回收铝,同时实现铝资源回收[19-21].沉淀回收铝后的溶液,还含有少量的有价金属,用于后续除铝完后的浸出液混合回收,实现资源循环利用.
1 试验药剂及原理方法
1.1 实验药剂
实验所用药剂为氢氧化钠、硫酸等均为分析纯.
1.2 实验仪器
实验仪器采用DF-101S集热式磁力搅拌器(江苏金坛市亿通电子有限公司)、SHB-B95型循环水式多用真空泵(巩义市予华仪器有限责任公司)、FA224电子天平(上海舜宇恒平科学仪器有限公司)、CHB-6020真空干燥箱(上海新苗医疗器械制造有限公司)、PHS-3E pH计(上海仪电科学仪器股份有限公司)、扫描电子显微镜(美国FEI公司)、X射线荧光光谱仪(爱丁堡).
1.3 实验原料
实验所用的锂离子电池原料全部来自于赣州市某厂的锂离子电池.经预放电、粉碎、干燥、研磨后,经过XRF的初步检测,废料中的主要金属元素有12.441 %的Al,10.848 %的Cu,32.389 %的Co,以及1.014 %的Ni.
1.4 实验原理及实验流程
在钴酸锂电池中铝片大多数以铝单质的形式存在,废旧电池粉内铝为两性元素能溶于氢氧化钠溶液,在钴酸锂电池废料中钴酸锂在氢氧化钠溶度过高时,少量的钴酸锂会溶解,废旧钴酸锂电池废料里面一些微量化合物在不同的碱溶度下会发生少量的溶解反应.其余金属化合物基本上难溶于氢氧化钠溶液.发生的主要反应如下:为了将铝去除和回收并降低成本,本实验采用二级逆流碱浸法,其工艺流程如图 1所示.
1.5 实验步骤
1) 一级碱浸:称取NaOH固体加入上一组二级碱浸的滤液,保持一定的固液比,分5次每次间隔5 min加入锂离子电池废料.在一定温度下反应一定时间,过滤,滤渣进行二级碱浸.
2) 称取NaOH固体,配制成一定浓度的NaOH溶液.摇匀后备用.
3) 二级碱浸:根据配比计算后,取一定量固定浓度的NaOH溶液加入到上述1)的碱浸渣中,在一定温度下反应一定时间后过滤,滤液进入下一组实验.
4) 往步骤1)所得滤液中以一定流量加入硫酸溶液,保持一定的搅拌强度和温度,待溶液pH值到达设定值时,停止加硫酸溶液,过滤该溶液,得到氢氧化铝沉淀.
1.6 锂离子电池废料的浸出率计算
称取一定质量的锂离子电池废料,其质量记为M1,在一定条件下向废料中加入一定体积和浓度的NaOH碱溶液进行二级逆流浸出.反应一定时间后过滤、洗涤,在烘箱中干燥2 h后进行称重,干燥后重量记为M2.

将干燥后的滤渣再次加入NaOH中,在一定条件下进行二级碱浸,反应一定时间后过滤、洗涤,在烘箱中干燥2 h后称重,干燥后的质量记为M3.
二级碱浸浸出率


2 结果与讨论
2.1 各类因素对锂电池废料浸出率的影响
2.1.1 加料方式对浸出率的影响
加料方式一般有2种,先加入固体锂离子电池废料再加入NaOH溶液,先加入NaOH溶液再加入固体锂离子电池废料.
为使固体与液体两相充分接触,本实验采用连续加料的方式,即每次向液体中加入2.000 0 g废料,每5 min加1次,或向固体中加入一定体积的NaOH溶液,每5 min加1次.
在不知道最合适条件的情况下,首先假定一组实验.设定实验一级碱浸固液比1:10,NaOH用量为2.500 0 g,锂离子电池废料10.000 0 g,温度为60 ℃,二级碱浸浓度为10 %(100 g/L),碱配比4:6,反应时间为1 h.模拟不同加料方式对一二级碱浸浸出率的影响.结果如表 1所列.二级碱浸因为加料不方便等原因采用一次性加料完成的方式.
表 1 加料方式与浸出率的关系Table 1. The relationship between feeding method and dissolution rate
如表 1可以看出,一级碱浸加料方式先加碱或是先加料对于碱浸效果无太大影响,但是先加碱液后加固体废料浸出率更高一些,这是因为NaOH与Al反应是为放热反应,在反应过程中约有2 ℃的温度升高,此热量会随着每一次加料而产生并加热NaOH,维持体系自放热时间长,而且在此过程中碱一直保持过量的状态,所以一级碱浸采用先加NaOH后加料的方式比较好.
二级加料方式中先加料后加碱的加料方式比先加碱后加料要好,因为NaOH与废料的固液比很小,先加液体再加固体可能会导致粉末状废料有部分黏附在烧杯壁上,没有参与反应,并且反应过程中原料一直处于很黏稠的状态,反应不充分.
2.1.2 反应温度对浸出率的影响
选择NaOH总量为2.500 0 g,加入废料为10.000 g,一级碱浸固液比1:10(g/L),二级碱浸浓度为10 %(100 g/L),一二级碱浸碱配比为4:6,反应时间为1 h.一级碱浸加料方式为先加碱液后加废料,每5 min加2.000 0 g,二级碱浸加料方式为先加废料再加碱液.设置不同的反应温度分别为40 ℃、50 ℃、60 ℃、70 ℃、80 ℃,试验结果如图 2所示.
由图 2可以得到,一级碱浸浸出率随着温度增高而增大,这是因为虽然NaOH和铝反应是放热反应,但是一级碱浸固液比大,反应自放热对一级碱浸升温效果几乎可以忽略不计,且温度越高,分子热运动就会加剧,从而增加了活化的分子数,溶液中分子间碰撞几率增大,反应程度增加.但是当温度高到一定程度的时候,铝几乎反应完全,所以浸出率无法再进一步增高.但是二级碱浸铝浸出率跟温度无太大关系,这是因为二级碱浸NaOH浓度大,固液比小,从而自放热对反应影响比一级碱浸出要大.
2.1.3 反应时间对浸出率的影响
选择NaOH量为2.500 0 g,加入废料为10.000 0 g,一级碱浸固液比1:10,二级碱浸浓度为10 %(100 g/L),一二级碱浸碱配比为4:6.一级碱浸加料方式为先加碱液后加废料,每5 min加2.000 0 g,二级碱浸加料方式为先加废料后加碱液.设置反应温度为80 ℃,分别设置反应时间为0.5 h、1 h、1.5 h、2 h、2.5 h来探究反应时间对铝浸出率的影响.试验结果如图 3所示.
从图 3中可以看出,随着时间的增加,浸出率虽然一直在增加,在1.5 h之后浸出率趋于平缓,从1.5~2 h的增加量远没有从0.5 h到1 h的增加量大.综合许多成本和生产因素,所以最合适浸出时间应该选择1 h.
2.1.4 二级碱浸碱浓度对浸出率的影响
选择NaOH量为2.500 0 g,加入废料为10.000 0 g,一级碱浸固液比1:10,二级碱浸浓度分别为2 %、5 %、7.5 %、10 %、12 %、15 %,一二级碱浸碱配比为4:6.一级碱浸加料方式为先加碱液后加废料,每5 min加2.000 0 g,二级碱浸加料方式为先加废料后加碱液,温度为80 ℃,反应时间为1 h.探究不同NaOH浓度下对二级碱浸铝浸出率的影响.试验结果如图 4所示.
由图 4可得,随着二级碱浸浓度的增长,二级碱浸铝浸出率会先降低后增高.当二级碱浸碱液浓度为5 %时浸出率最高.这是因为在已经固定了一二级碱浸总碱量和碱配比的情况下,二级碱浸碱浓度是和固液比成反比的.浓度越小,固液比越大.于是当浓度低时,可能是固液比增大从而影响了浸出率,而当浓度高时主要是固液比过小从而影响了浸出率.
2.1.5 一级碱浸固液比对浸出率的影响
选择NaOH量为2.500 0 g,加入废料为10.000 0 g,一级碱浸固液比分别选择1:2、1:5、1:8、1:10、1:12、1:15,二级碱浸浓度为10 %(100 g/L),一二级碱浸碱配比为4:6.一级碱浸加料方式为先加碱液后加废料,每5 min加2.000 0 g,温度为80 ℃,反应时间为1 h.探究了在不同固液比的条件下对一级碱浸铝浸出率的影响.试验结果如图 5所示.
如图 5所示,如同实验二级碱浸浓度对浸出率的影响一样,随着固液比的加大,浸出率先增加后减小,和上一实验结论一样,在已经固定了一二级碱浸总碱量和碱配比的情况下,一级碱浸固液比和碱液浓度是成反比的.固液比大的时候主要是浓度减小主要限制了浸出率,而固液比小的时候主要是固液比限制了浸出率,一级碱浸合适的固液比为1:12.
2.1.6 一、二级碱浸碱配比对浸出率的影响
选择NaOH量为2.500 0 g,加入废料为10.000 0 g,一级碱浸固液比分别选择1:12,二级碱浸浓度为10 %(100 g/L),一二级碱浸碱配比为分别设置为0:10、2:8、4:6、6:4、8:2.一级碱浸加料方式为先加碱液后加废料,二级碱浸方式为先加料后加碱液,废料每5 min加2.000 0 g,温度为80 ℃,反应时间为1 h.试验结果如图 6所示.
结果分析:随着第一级碱浸占总碱量的增加,第一级浸出铝浸出率增加而第二级碱浸铝浸出率减小.这是因为在固定了第一级碱浸固液比和第二级碱浸的浓度,以及总碱量的情况下,第一级碱浸配比增加势必会使第一级碱浸碱液浓度增大而使第二级碱浸碱液固液比减小,而总浸出率的变化规律则是先增加后减小.当一二级碱浸配比为6:4时,浸出率最大,为12.17 %.
2.1.7 一、二级碱浸总碱量对浸出率的影响
因为Al与NaOH反应生成的NaAlO2要使NaOH要过量.因此本实验在一开始就从Al的化学计量比的1.1倍的NaOH开始向上增加.
选择NaOH量为12.441 %原料所含Al化学计量比的1.1倍(2 g)、1.2倍(2.2 g)、1.3倍(2.4 g)、1.4倍(2.57 g)、1.5倍(2.76 g),加入废料为10.000 0 g,一级碱浸固液比选择1:12,二级碱浸浓度为5 %(50 g/L),一二级碱浸碱配比为分别设置为6:4.一级碱浸加料方式为先加碱液后加废料,二级碱浸方式为先加料后加碱液,废料每5 min加2.000 0 g,温度为80 ℃,反应时间为1 h.试验结果如图 7所示.
根据图 7得出随着加入的总碱量的增加,浸出率也随着增加,但是趋势趋于平缓,比如当NaOH化学计量比为Al的1.4倍的时候,浸出率达到了12.25 %,而NaOH用量增大到1.5倍的时候,浸出率只增长了一点点为12.32 %,这将近达到了铝的全部的量,所以NaOH加入量为锂离子电池废料中Al化学计量比的1.5倍时是较好的.如果考虑到实际生产,则较优情况是1.4倍.
2.1.8 浸出段数对浸出率的影响
实验中以NaOH量为2.500 0 g,加入废料为10.000 0 g,一级碱浸固液比分别选择1:12,二级碱浸浓度为10 %(100 g/L),一二级碱浸碱配比分别设置为6:4.一级碱浸加料方式为先加碱液后加废料,二级碱浸方式为先加料后加碱液,废料每5 min加2.000 0 g,温度为80 ℃,反应时间为1 h的实验组为二段浸出的实验,将一级碱浸浸出液和二级碱浸浸出液混合当做三级碱浸液来实验三段碱浸对铝浸出率的影响,第三段碱浸中所用的NaOH完全来自前两级碱浸浸后液,不添加新的碱,反应温度为80 ℃、时间为1 h、加料方式为先加碱液后加料.试验结果见表 2.
表 2 碱浸出段数对浸出量的影响Table 2. Effect of alkali solution number on dissolution rate
该结果说明二段碱浸已经能浸出大部分的铝,为了节约费用和时间,建议采用二段浸出的方式来浸出锂离子电池废料中的铝.
2.2 碱浸溶液回收氢氧化铝
2.2.1 pH对氢氧化铝沉淀的影响
取同一烧杯中充分混匀的一级碱溶液5份,每份各100 mL,配置体积浓度为10 %的硫酸溶液和质量浓度为10 %的NaOH溶液作为调节pH值的作用,向碱溶液中缓缓滴入稀硫酸,边搅拌边用pH计测定pH值,在pH值分别为6、7、8、9时,再过滤、洗涤、干燥.试验结果见表 3.
表 3 不同pH值下铝的沉淀量Table 3. Precipitated aluminum at different pH
pH越大,其中固体颗粒就越大,干燥后就越难被磨碎,同时颜色也越偏黄色,可以用液相中固体颗粒长大的吸附层理论及扩散理论来证实.所以一级碱浸浸出液沉淀氢氧化铝的最合适pH值为7~8.
而pH小于5的时候生成的沉淀会逐渐消失,这是因为酸性强而导致氢氧化铝与酸反应.
2.2.2 氢氧化铝化学组成
沉淀产生的氢氧化铝的XRF检测结果如表 4所列.
表 4 沉淀出的氢氧化铝的化学组成/(质量分数,%)Table 4. Chemical composition of precipitated aluminum hydroxide /(mass fraction, %)
显示其中含有大量的Al元素而几乎不含Cu、Co、Ni等元素,说明其沉淀产物较多为Al.
2.2.3 氢氧化铝产品表征
图 8所示为沉淀产生的氢氧化铝扫描电镜的形貌.
图 9所示为沉淀产生的氢氧化铝扫描电镜的形貌.
可以看到其中含有团聚在一起的,直径约为40 μm的颗粒,在颗粒之上还有更加细小的粉末.其原因为:氢氧化铝的等电位点约为pH值在7.5~8.5之间,且氢氧化铝胶体颗粒在等电位附近的粒间静电斥力会变弱,在结晶过程中无法避免地会发生絮状颗粒的增大.
3 结论
1) 一级碱浸先加碱液后加固体废料,二级碱浸先加固体后加碱液,并且分段加入,浸出率比较高.
2) 二级逆流碱浸较适宜条件为:加入总碱量为Al化学计量比的1.5倍,碱配比为6:4,一级碱浸固液比为1:12,二级碱浸浓度为5 %,反应时间为1 h,温度80 ℃.在该条件下废旧锂离子电池废料中铝的浸出率为12.32 %.
3) 从一级碱浸滤液中沉淀氢氧化铝的合适pH值为7~8.
-
-
[1] XU Y J, LI G X, SUN Z Y. Development of biodiesel industry in China: Upon the terms of production and consumption[J]. Renewable and Sustainable Energy Reviews, 2016, 54: 318-330. doi: 10.1016/j.rser.2015.10.035
[2] SULTANA N, PATHAK A K, GURIA C. Response surface method and genetic algorithm assisted optimal synthesis of biodiesel from high free fatty acid sal oil (Shorea robusta) using ion-exchange resin at high temperature[J]. Journal of Environmental Chemical Engineering, 2015(3): 2378-2392. https://www.researchgate.net/publication/283434072_Response_surface_method_and_genetic_algorithm_assisted_optimal_synthesis_of_biodiesel_from_high_free_fatty_acid_sal_oil_Shorea_robusta_using_ion-exchange_resin_at_high_temperature
[3] OLKIEWICZ M, PLECHKOVA N V, EARLE M J, et al. Biodiesel production from sewage sludge lipids catalysed by Br$\phi $nsted acidic ionic liquids[J]. Applied Catalysis B: Environmental, 2016, 181: 738-746. doi: 10.1016/j.apcatb.2015.08.039
[4] ANDRE G B, VIRGINIA P. Organic municipal solid waste (MSW) as feedstock for biodiesel production: A financial feasibility analysis[J]. Renewable Energy, 2016, 86: 1422-1432. doi: 10.1016/j.renene.2015.08.025
[5] PENG B X, SHU Q, WANG J F, et al. Biodiesel production from waste oil feedstocks by solid acid catalysis[J]. Process Safety and Environmental Protection, 2008, 86: 441-447. doi: 10.1016/j.psep.2008.05.003
[6] THEAM K L, ISLAM A, Choo Y M, et al. Biodiesel from low cost palm stearin using metal doped methoxide solid catalyst[J]. Indu-strial Crops and Products, 2015, 76: 281-289. doi: 10.1016/j.indcrop.2015.06.058
[7] RUTH G, PIYALI B, ASIM B. Sulfonated porous organic polymer as a highly efficient catalyst for the synthesis of biodiesel at room temperature[J]. Journal of Molecular Catalysis A: Chemical, 2016, 411: 110-116. doi: 10.1016/j.molcata.2015.10.016
[8] BARBARA S C, CATIA S N, PAULO R S, et al. Supercritical ethanolysis for biodiesel production from edible oil waste using ionic liquid [HMim][HSO 4] as catalyst[J]. Applied Catalysis B: Environmental, 2016, 181: 289-297. doi: 10.1016/j.apcatb.2015.07.047
[9] GONG S W, LU J, WANG H H, et al. Biodiesel production via esterification of oleic acid catalyzed by picolinic acid modified 12-tungstophosphoricacid[J]. Applied Energy, 2014, 134: 283-289. doi: 10.1016/j.apenergy.2014.07.099
[10] ZOU C J, ZHAO P W, SHI L H, et al. Biodiesel fuel production from waste cooking oil by the inclusion complex of heteropoly acid with bridged bis-cyclodextrin[J]. Bioresource Technology, 2013, 146: 785-788. doi: 10.1016/j.biortech.2013.07.149
[11] AMIN T K, NOR A S A, ALIREZA Z, et al. Transesterification of waste cooking oil by heteropoly acid (HPA) catalyst: Optimization and kinetic model[J]. Applied Energy, 2013, 102: 283-292. doi: 10.1016/j.apenergy.2012.07.018
[12] 舒庆, 刘宝, 宋胜海, 等. 钨钼混配型杂多酸盐催化剂上酯化反应条件的优化研究[J].有色金属科学与工程, 2014(6): 521-27. http://ysjskx.paperopen.com/oa/DArticle.aspx?type=view&id=201406004 [13] NAONOBU K, TSUBASA H, MITSUO O, et al. Biodiesel production using heteropoly acid-derived solid acid catalyst H 4PNbW 11O 40/ WO 3-Nb 2O 5[J]. Applied Catalysis A: General,2009, 363: 164-168. doi: 10.1016/j.apcata.2009.05.012
[14] NILESH N, VARSHA B, ANJALI P. Efficient synthesis of biodiesel from waste cooking oil using solid acid catalyst comprising 12-tungstosilicic acid and SBA-15[J]. Fuel, 2014, 135: 253-261. doi: 10.1016/j.fuel.2014.06.062
[15] VARSHA B, ANJALI P. 12-Tungstophosphoric acid anchored to SBA-15: An efficient, environmentally benign reusable catalysts for biodieselproduction by esterification of free fatty acids[J]. Applied Catalysis A: General, 2011, 403: 161-172. doi: 10.1016/j.apcata.2011.06.027
-
期刊类型引用(0)
其他类型引用(1)




 下载:
下载: