Causticizing-precipitating scheelite by calcium hydroxide from sodium tungstate solution
-
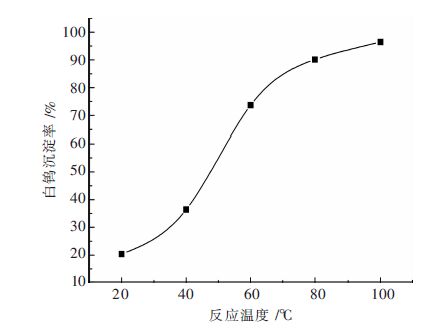
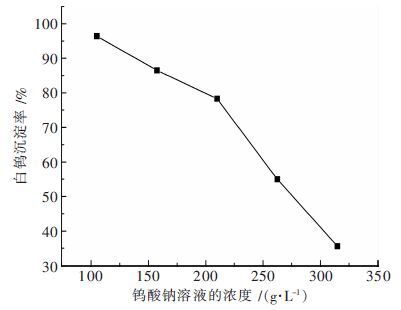
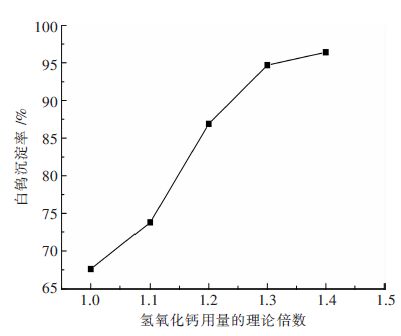
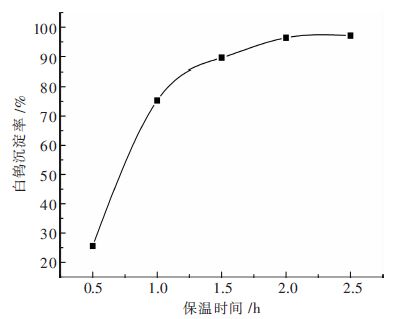
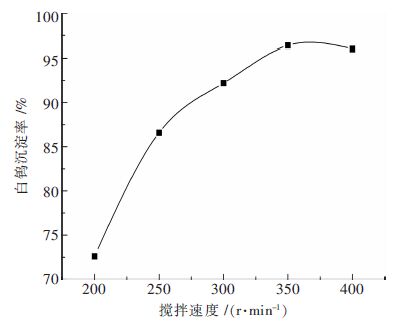
摘要: 钨酸钠溶液氢氧化钙苛化-沉淀白钨工艺是实现钨矿NaOH分解试剂回收,以至黑钨和黑白钨混合矿绿色冶炼的技术途径;研究了不同温度下钨酸钠溶液氢氧化钙苛化-沉淀白钨反应的平衡常数和标准自由能变化,分析了氢氧化钠和钨酸钠的活度系数对反应平衡的影响;工艺研究表明:温度和钨酸钠溶液浓度对白钨沉淀率的影响非常显著.当氢氧化钙用量为1.4倍理论量,温度100 ℃,钨酸钠溶液钨浓度为105 g/L,保温时间为2 h,搅拌速度为350 r/min,白钨沉淀率可达96 %以上.与传统工艺采用氯化钙作为沉淀剂相比,氢氧化钙沉淀白钨所需的理论量倍数较大、反应的时间较长、搅拌速度更快.Abstract: The process of causticizing -precipitating scheelite by calcium hydroxide from sodium tungstate solution is the technical way of the realization of recycling NaOH reagent for tungsten ore decomposition ,and wolframite and scheelite and wolframite mixed ore green smelting. This paper studies equilibrium constant and standard free energy change of causticizing -precipitating scheelite by calcium hydroxide from sodium tungstate solution reaction at different temperatures, and analyzes the effect of the activity coefficient of sodium hydroxide and sodium tungstate on the reaction equilibrium. The research shows that the effect of temperature and sodium tungstate solution concentration on precipitation rate of scheelite is very significant. When the calcium hydroxide dosage is 1.4 times of the theoretical amount, temperature 100℃, tungsten concentration of sodium tungstate solution is 105 g/L, the holding time is 2 h, the stirring speed is 350 r/min, the precipitation rate of scheelite can reach above 96%. Comparing with traditional process of calcium chloride used as a precipitating agent, calcium hydroxide precipitating scheelite requires larger times of the theoretical amount, longer reaction time and faster stirring speed .
-
Keywords:
- sodium tungstate solution /
- calcium hydroxide /
- caustic /
- precipitate /
- scheelite
-
0 引言
非晶态合金(金属玻璃)具有连续的无定形结构缺陷,其组织均匀,不存在位错、晶界、孪晶界等,因此具有高的弹性模量、强度、硬度、耐磨和耐腐蚀性等优点,但目前对于非晶合金的研究大多是粉末或薄带材料,大块非晶合金很少.
非晶不存在晶粒、无磁各向异性和钉扎磁畴壁的缺陷,因此具有比广泛应用的硅钢和Fe-Ni坡莫合金更高的磁感、磁导率和较低的铁损、矫顽力[1-4].
Al75Fe12.5V12.5非晶相对于晶态铝基合金拥有优异的耐蚀、耐磨、力学和电磁特性,部分晶化后的强度可与工程陶瓷媲美[5-6],正逐步成为电力、精密机械等领域重要的功能材料.
1 实验材料及方法
使用纯度均为99.9 %,粒径分别为:Al 74 μm,V 74 μm,Fe 100 μm,采用机械合金化法在行星式球磨机中制备非晶.根据Al75Fe12.5V12.5的原子百分比,称量Al、Fe、V粉共20 g并混匀,与0.4 g硬脂酸(C18H36O2)和400 g轴承钢珠装入球磨罐内后带上螺钉,移入真空手套箱并抽真空至10-6 Pa后冲入高纯Ar气,球磨过程中,每5 h取样一次.
2 实验结果及分析
2.1 Al75Fe12.5V12.5合金粉的非晶化
经球磨70 h后,Al、Fe和V及未知固溶体相的晶态衍射峰完全消失,在XRD图 37°~46°范围出现漫散峰,表明此时组元间完全互溶,合金粉末处于完全非晶状态.Al75Fe12.5V12.5的机械合金化过程可以表述为:元素粉末→Al基固溶体+金属间化合物(Al45V7,Al5Fe2)→非晶相.Al75Fe12.5V12.5合金粉的TEM图及对应SEAD衍射图如图 1,由SEAD衍射图可见,仅有弥散的晕环,不存在晶体特有的对称衍射斑点,说明非晶化过程完成.
称取4 g Al75Fe12.5V12.5非晶粉末进行SPS烧结,制得块体非晶合金,SPS固结条件为:压力50 MPa,保温6 min,升温速率100 ℃/min,烧结温度分别为420 ℃、450 ℃、500 ℃.
2.2 块体非晶合金的宏观形貌与物相分析
非晶合金在玻璃态转变温度Tg以下时效会发生结构弛豫,材料内部结构发生局部调整,导致区域应力部分松弛.图 2分别为SPS在420 ℃、450 ℃、500 ℃下的块体Al75Fe12.5V12.5合金的XRD图,可见,在420 ℃烧结时,由于结构弛豫,块体非晶中析出了少量的Al76.8Fe24相,形成非晶/纳米晶复合组织.在450 ℃烧结,得到XRD图谱中只有单一的漫散峰,无晶体相析出,合金为完全非晶状态[7-10].将烧结温度升高到500 ℃时,样品材料中出现了多晶体衍射峰,材料开始晶化,析出Al5Fe2和Al3V相.因此,SPS烧结温度以450 ℃为宜.
图 3为4 g重的Al75Fe12.5V12.5非晶粉末,在50 MPa、450 ℃保温6 min,升温速率为20 ℃/min的条件下采用SPS烧结,得到尺寸为ϕ20 mm×2 mm的块体非晶合金的金相照片.计算出密度为2.93 g/cm3,块体试样表面比较粗糙,没有金属光泽,其在高倍下的颗粒较松散,结合不紧密,表明块体致密度不高,这可能与SPS烧结时,所加载的压力较小有关.
2.3 块体合金的物性研究
将非晶试样磨抛后,置于MHV-1000显微维氏硬度计上,每一试样在5个不同区域依次施加0.49 N、0.98 N、1.96 N、......、直到4.9 N载荷并保载15 s后,测量其维氏硬度值,其平均值即为试样的对应载荷的硬度值.
块体Al75Fe12.5V12.5非晶在500 ℃保温30 min后的维氏硬度值与力值关系曲线如图 4.由图 4可知,硬度值随载荷增加而减小,载荷小于1.96 N时,硬度降低较快,曲线斜率较大.曲线在1.96 N载荷处产生突变,随后曲线斜率变小,这可能是由试样的微裂纹和压痕尺寸效应造成[4-7].显微维氏硬度值随负荷的增加而减小即为压痕尺寸效应,载荷小于1.96 N时,试样的压痕尖角处无微裂纹出现,但压痕附近有较高应力集中,负荷卸载后压痕尺寸收缩较大,使得压痕尺寸效应明显;载荷大于1.96 N时,微裂纹在压痕尖角处形成,并沿能量耗散最小的方向迅速扩展,在压痕尖角处产生崩裂,使压痕尖角处的应力集中部分得到释放[11-12].因此,0.98 N是块体Al75Fe12.5V12.5非晶的最佳显微维氏硬度负荷,此负荷对应的显微维氏硬度值是准确的.
理论情况下,压痕对角线的长度与载荷大小均不影响显微维氏硬度值.实际上,由于金属存在弹性回复,压头会在某一负载下在试样表面产生的压痕当缷载后收缩变小.金属的弹性回复仅与其种类有关,与压痕对应的负荷无关.在低负荷(小于1.96 N)时,产生的很小压痕会收缩很大比例,使得所测显微维氏硬度值偏高.
图 5为Al75Fe12.5V12.5非晶试样断面的宏观形貌,由图 5可见,断口呈放射花样,不存在塑性变形的韧窝形貌,说明Al75Fe12.5V12.5非晶断裂属于脆性断裂.
在纯铝粉中添加不同Al75Fe12.5V12.5非晶粉末含量,组成5 g混合粉末并混匀后,在表 1条件下采用放电等离子烧结,得到复合材料的形貌如图 6.可见,复合块体金属光泽度随非晶含量增加而降低,这与非晶长程无序的结构有关.
表 1 放电等离子烧结试验条件Table 1. Test conditions of plasma sintering试验条件 试验载荷
/kN升温速率
/(℃·min-1)加热温度
/℃保温时间
/min参数值 15 20 500 5 与图 6中非晶含量对应的复合材料金相图如图 7,由图 7可见,非晶增强体进入基体铝的晶界,使得这些区域成为晶体-非晶强化界面,随非晶粉末含量的增加,基体铝晶界间隙慢慢变宽直至消失[13-15].表 2为不同非晶含量的复合材料密度,可见,复合材料的密度随非晶含量增加而增大,但小于完全非晶态Al75Fe12.5V12.5的密度2.93 g/cm3.
表 2 不同非晶含量的复合材料密度Table 2. Density of amorphous composite非晶含量/% 0 10 30 50 70 90 ρ /(g·cm-3) 2.45 2.53 2.57 2.60 2.66 2.79 根据块体非晶合金样品的特点,采用显微维氏硬度法测量其硬度值,为确定显微维氏硬度所需加载的负荷大小及硬度与非晶含量的关系,对块体复合材料加载不同载荷,得到如图 8所示不同非晶比例的复合材料显微维氏硬度与负荷关系图,由图 8可见,复合材料的硬度值如同完全非晶材料,都随负荷的增大而降低,且负荷在1.96 N以下时硬度-负荷曲线斜率较大,负荷在1.96 N以上时硬度-负荷曲线斜率较小,曲线在1.96 N处发生突变,压痕尖角裂纹以散射形式扩展[16].负荷为0.49 N时,非晶含量为10 %的复合材料显微维氏硬度为HV0.05=94.3,较晶体铝的显微维氏硬度HV0.05=34.8有明显提高,这是由于复合材料中含有晶体-非晶强化界面,基体铝能提供与非晶接触的晶界数量有限,这就限制了形成晶体-非晶强化界面的数量,因此,复合材料的显微维氏硬度值会随非晶含量增加有所提高,但不明显.
3 结论
1)以6061粉为基体,按一定配比加入Fe、V微粉,球磨70 h后可得到约为23 nm的Al75Fe12.5V12.5完全非晶粉末.
2)不同非晶含量的复合材料,非晶增强体进入基体铝的晶界,使得这些区域成为晶体-非晶强化界面,随非晶粉末含量的增加,基体铝晶界间隙慢慢变宽直至消失.
3)非晶粉末在450 ℃烧结,得到的XRD图谱中只有单一的漫散峰,无晶体相析出,合金为完全非晶状态,SPS烧结温度以450 ℃为宜.
4)由于压痕尺寸效应和微裂纹,复合材料的硬度值如同完全非晶材料,都随负荷的增大而降低,且负荷在1.96 N以下时硬度-负荷曲线斜率较大,负荷在1.96 N以上时硬度-负荷曲线斜率趋于缓和,压痕尖角裂纹以散射形式扩展.
-
表 1 氢氧化钙和鸽酸钙的溶度积

表 2 反应的标准自由能变化àG1 及平衡常数Ka

表 3 Na2OH 溶液在70 "c下的平均活度系数

表 4 Na22WO4 溶液在252 "c下的平均活度系数

-
[1] 万林生,赵奎,羊建高,等. 钨冶金[M].北京:冶金工业出版社,2011:49. [2] 万林生,邓登飞,赵立夫,等.钨绿色冶炼工艺方向和技术进展[J].有色金属科学与工程,2013,4(5):15-18. http://ysjskx.paperopen.com/oa/DArticle.aspx?type=view&id=2013050004 [3] 万林生,赵立夫,李红超.章源钨业APT绿色冶炼的技术进步和发展[J].中国钨业,2012,279(1):23-26. http://www.cnki.com.cn/Article/CJFDTOTAL-ZGWU201201012.htm [4] 万林生,徐国钻,严永海,等.中国钨冶炼工艺发展历程及技术进步[J].中国钨业,2009,24(5):63-66. http://www.cnki.com.cn/Article/CJFDTOTAL-ZGWU200905017.htm [5] 万林生,赵立夫,黄泽辉,等.一种铵盐分解白钨矿的方法:中国,ZL201110063533.0[P].2011-08-17. [6] 范树森.钨酸钙的粒度控制及白钨母液中钨的回收[J].稀有金属与硬质合金,1993(2):13-16. http://www.cnki.com.cn/Article/CJFDTOTAL-XYJY199302003.htm [7] 李裕芳,赖道玉.不同颗粒人造白钨的制取[J].中国钨业,1990(11):11-14. http://www.cnki.com.cn/Article/CJFDTOTAL-ZGWU199011002.htm [8] 李自强,何良惠.沉淀白钨的条件分析[J].稀有金属,1987(4):249-252. http://www.cnki.com.cn/Article/CJFDTOTAL-ZXJS198704002.htm [9] 路辉,谢刚,俞小花,等.仲钨酸铵结晶母液处理技术研究进展[J].稀有金属与硬质合金,2009,37(4):44-47. http://www.cnki.com.cn/Article/CJFDTOTAL-XYJY200904011.htm [10] 叶大伦,胡建华.实用无机物热力学数据手册[M].北京:冶金工业出版社,2002. [11] 王海川,董元篪.冶金热力学数据测定与计算方法[M].北京:冶金工业出版社,2005. [12] 李洪桂.钨矿物原料碱分解的基础理论及新工艺[M].长沙:中南工业大学出版社,1997. [13] 李运姣,李洪桂,刘茂盛.白钨矿碱分解过程的热力学和动力学研究[J].中国有色金属学报,1990(1):39-45. [14] 孙培梅,李运姣,李洪桂,等.白钨矿碱分解过程的热力学研究[J].中国有色金属学报,1993(2):37-43. http://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ199302010.htm [15] 李运姣,孙培梅,刘茂盛,等.白钨矿的机械活化碱分解[J].中国有色金属学报,1995(3): 27-29. http://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ503.006.htm




 下载:
下载: