Solvothermal preparation spherical ZnS nano-photocatalyst and its photocatalytic activity
-
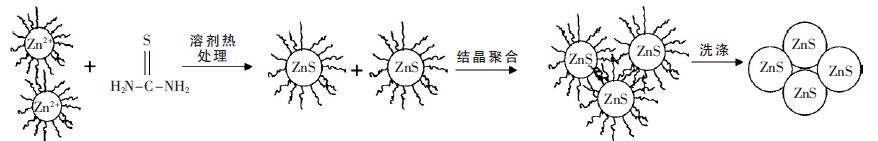
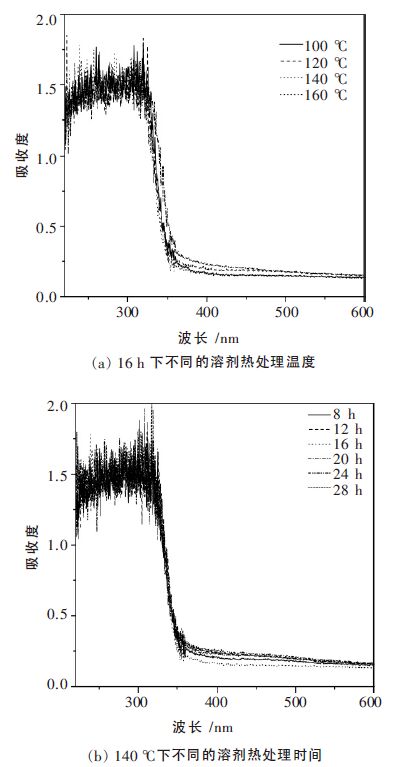
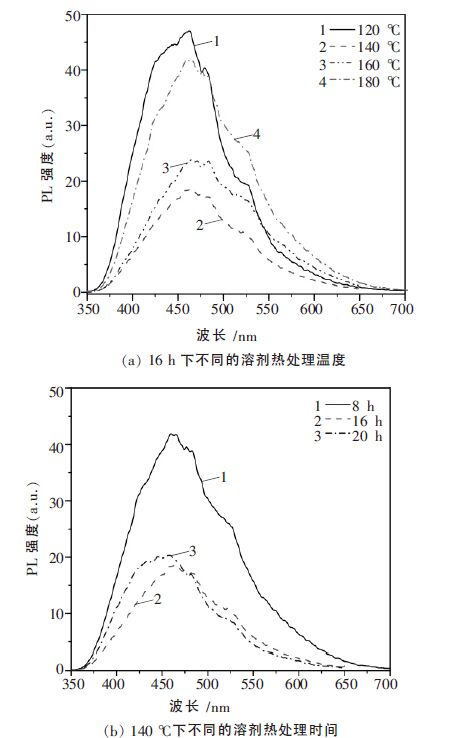
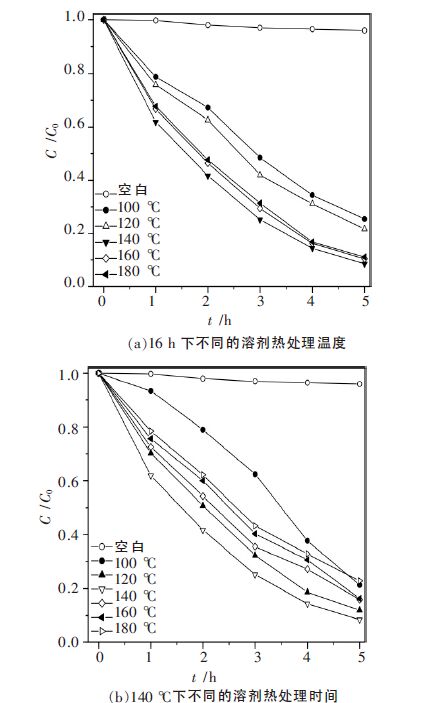
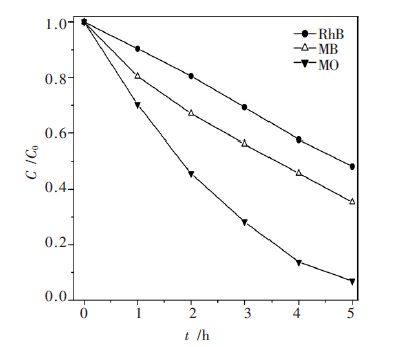
摘要: 以乙二醇为反应介质,醋酸锌(Zn(CH3COO)2·2H2O)和硫脲((NH2)2CS)为原料,采用溶剂热法制备了系列ZnS 光催化剂. 考察了不同反应温度、时间对ZnS 的结晶度、比表面积、光吸收性能及其光催化性能的影响. 利用N2物理吸附(BET)、扫描电镜(SEM)、X 射线衍射(XRD)、紫外可见漫反射光谱(UV-vis DRS)和光致发光谱(PL)对样品进行了表征.以紫外灯(λ=254 nm)为光源,对所得样品进行了光催化降解不同染料的活性测试.研究结果表明,该系列催化剂均具有较好的结晶性能,具有球状形貌.但在140 ℃下处理16 h 的ZnS 样品表现了最高的光催化活性. 光照5 h 后, 染料酸性橙Ⅱ、甲基橙、亚甲基蓝、罗丹明B 的降解率分别达到91.49 %、93.04 %、64.63 %和51.79 %.这主要归于该条件下制备的ZnS 具有比较好的结晶性能、大比表面积和较少的晶格缺陷.Abstract: A series of ZnS photocatalysts were prepared by a solvothermal method with ethylene glyclo as the reaction medium and zninc acetate (Zn (CH3COO)2·2H2O)and thiourea ( (NH2)2CS)as the precursors. The effects of reaction temperature and time on the crystallnity , surface area, optic adsorption and photocatalytic performance of the ZnS were investigated. The obtained ZnS samlpes were characterized by N2 physical adsorption (BET), scanning electron microscopy (SEM),X-ray diffraction (XRD),UV-vis diffuse reflectance spectra (UV-vis DRS), and photoluminescence spectroscopy (PL). The photocatalytic activity of the prepared samples was evaluated by photocatalytic degradation of different dyes under UV light (λ=254 nm) irradiation. The experimental results show that these photocatalysts prepared under solvothermal condition have good crystallinity and spherical morphology. ZnS photocatalyst prepared at 140 ℃ for 16 h shows the highest photocatalytic activity in decomposition of the dyes. After 5 hours ’ light irradiation, the degradation rates of the dyes of acid orange II, methyl orange, methylene blue, and rhodamine B are 91.49 %, 93.04 %, 64.63 % and 51.79 %, respectively. This high photocatalytic activity is mainly attributed to the good crystallnity , big surface area, and less crystal defects of the ZnS prepared under the optimal condition.
-
Keywords:
- ZnS /
- solvothermal /
- ethylene glyclo /
- photocatalytic /
- dyes
-
0 引言
地应力是存在于地壳中未被人工扰动的天然应力,其在指导矿山地下开采工程、土木建设工程、边坡修建等工程中的作用越来越重要,是各种工程开挖设计必须要做好的前提工作.国内外对获取初始地应力进行了大量研究,并取得了许多研究成果[1-2].目前初始地应力的计算方法大致有以下几种:边界荷载调整、应力函数法、有限元数学模型回归分析法、三维有限元反演分析法等[3-5].在地应力反演方法上,主要采用的是以分析开挖工程区域小范围内的初始地应力场的数学计算方法,包括:位移反分析方法,由监测位移反演局部区域的应力分布[6];应力反演方法,由现场有限个点的地应力实测值,通过应力函数或数值计算方法回归分析得到研究区域的应力.
本文在前人的研究成果基础上,假定初始地应力场主要由自重应力场和地质构造运动应力场两部分组成,将各运动模式分解成应力基本运动模式,并结合多元线性回归原理,采用Fortran编写相关程序,以及FLAC3D强大的数值模拟分析功能[7],以此建立地应力场反演多元线性回归模型,发展和完善地应力场反演方法.
1 多元线性回归反演理论
多元线性回归法反演初始地应力场是当前常用且比较精确的方法之一[8],其基本思路是:通过在岩体的局部位置进行实际测量得到一定的地应力值,然后根据应力与荷载之间存在的相互关系,分析研究得到岩体的应力测量信息,再采用多元线性回归法展开计算分析,以此来反演地应力场.
1.1 基本影响因素模拟
由于初始地应力主要受岩体自重和地质构造运动两方面影响,进而把初始地应力场认为主要由自重应力场和地质构造运动应力场两个部分组成[9],通过用FLAC3D对自重应力场和地质构造应力场进行数值模拟.通常情况下,地质构造运动应力场由以下5种子构造应力模式线性叠加而成:
(1)左右方向水平挤压构造运动产生的应力场;
(2)前后方向水平方向水平挤压构造运动产生的应力场;
(3)水平面内剪切构造运动产生的应力场;
(4)左右垂直平面内的竖向剪切构造运动产生的应力场;
(5)前后垂直平面内的竖向剪切构造运动产生的应力场.
通过以上分析,就可以从影响地应力的众多因素中提取出6个主要因素,便于建立模型.
1.2 多元线性回归模型
基于多元线性回归原理,把所要求的初始地应力场作为因变量,形成地应力的各种基本因素作用作为自变量,采用弹性工作状态下的线性叠加原理写出初始应力场的回归模型[6, 10]为:

(1) 式(1)即为回归方程,Ci为回归系数,n代表自重和构造运动基本运动模式的种类(数值模拟时亦可称为工况).式(1)中,σjk0为k测点j应力分量的初始地应力计算值;Ci(i=0, 1, 2, …, 6)为7个待估参数,分别代表每类运动模式(自重运动模式及子构造运动模式)引起的应力分量大小;σjk0、σjk1、σjk2、σjk3、σjk4、σjk5、σjk6分别为自重和5个子构造运动作用下的k测点j应力分量;ε为模型误差.
上述回归模型的基本假定是:
(1)ε是因变量σjk0的误差,是相互独立的随机变量,没有系统性,其数学期望全为零,即E(εn)=0;
(2)每次观测相互独立,并有相同的精度,即εn之间的协方差关系式为

(3)εn服从正态分布.
1.3 回归系数Ci的确定
在使用回归方法反演区域应力场时,记第k个测点的实测地应力j分量值为





(2) 运用最小二乘法原理,使得残差平方和为最小值,即式(2)对Ci取偏导数并令其为零,则

(3) 对式(3)整理后得回归系数Ci的线性方程组矩阵:


由此方程组可求解出回归系数,它的解是唯一的,可以得到n+1个待定回归系数C=(C0,C1, C2,…,Cn)T.根据各基本运动模式(计算工况)应力回归方程,反演研究区域内任意点的应力,从而得到反演后区域内的整体应力场空间分布.
2 算例分析
为了验证上述所提出的初始地应力场反演与构建方法的可行性,利用Fortran语言编写了多元线性回归初始地应力场反演程序,并基于Flac3D有限差分软件,对一个简单平面应变问题的初始地应力场进行反演与构建.
如图 1所示为一地质剖面计算模型,模型左侧施加P=3.0MPa的均布荷载,右侧水平约束底部垂直约束,材料均质,弹性模量E=28GPa,泊松比μ=0.23,容重r=2.8×103kg / m3.
算例在方案设计时,选取如图 1所示计算模型计算区域内的6个点(A, B,…,F)作为假想的初始地应力实测点,各点在模型中相对应的单元编号分别为1070、1080、670、732、320、332.首先在Flac3D中按算例模型边界条件及自重条件下计算得到如表 1所示6个观测点的应力值,这些应力值作为各测点的初始地应力实测应力值;然后将自重因素视为未知因素,构造运动基本运动模式采用应力边界条件基本模式,分别计算单位自重、模型左侧边界单位均匀分布载荷、三角形分布载荷和剪应力分布载荷等4种工况的基本运动模式的应力场,再由这些已知的地应力值,利用所编写的多元线性回归初始地应力场反演程序,得到各工况基本运动模式的回归系数.至此,可根据各基本运动模式(计算工况)应力回归方程,反演研究区域内任意点的应力,从而得到反演后区域内的整体应力场空间分布.
表 1 地应力实测值
表 2列出了各运动模式单位荷载作用下应力回归系数,实际只考虑了自重和均布左侧边界加载,对模型的外部实际加载与反演加载相对误差分别为0.103%和1.4433%,计算表明反演非常成功,尤其是自重因素对形成初始地应力影响极其稳定.
表 2 各运动模式单位荷载作用下应力回归系数
图 2列出了各测点实测应力值与反演应力值,通过比较可以看出,各测点σx和σz实测应力值与反演应力值相对误差极小,均在0.5%以下,而剪应力的实测应力值与反演应力值误差略大一些,但相对误差也均在2.2%以下,由于区域的应力以水平和竖直方向为主,从工程的角度而言是可以接受的.
由此说明:反演应力与“实测应力”吻合较好,回归效果理想,采用多元回归方法反演线弹性地应力场是可行的,并且有较高的精度.
3 结论
(1)基于多元线性回归原理,把所要求的初始地应力场作为因变量,形成地应力的自重基本因素和构造运动的子构造应力基本因素作为自变量,采用线性叠加原理形成因变量与自变量的表达关系式,建立了多元线性回归地应力场反演数学模型,完善了多元线性回归地应力场反演方法.该方法保证了解的唯一性,使引进和剔除形成初始地应力场的各种因素有了根据,有利于对形成初始地应力复杂因素的认识.
(2)多元线性回归地应力场反演方法数学推导严密、物理意义明确,直接针对形成应力场的物理成因展开模拟,考虑了自重、挤压构造运动及剪切变形构造运动等共同作用.标准算例验证表明,计算应力值与观测应力值极其吻合,相对误差均在2.2%以下,回归效果理想,说明本文的地应力场反演方法可行,计算程序可靠,并且有较高的计算精度.
-
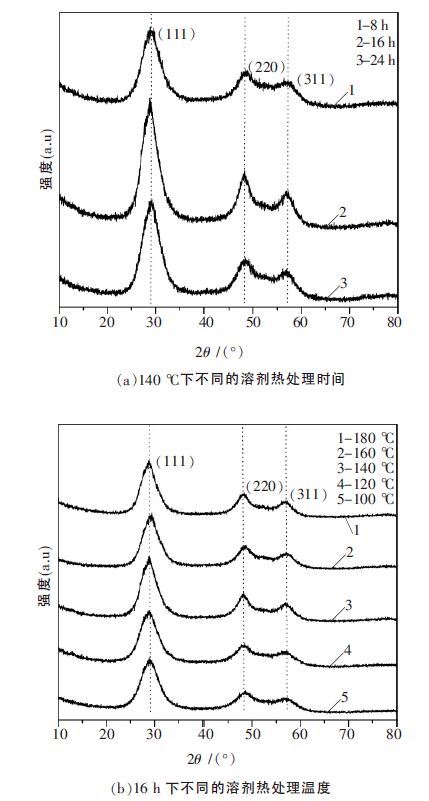
表 1 不同溶剂热条件下制备的ZnS 样品的比表面积

表 2 不同溶剂热条件下制备的ZnS 样品平均晶粒粒径

-
[1] 余长林,操芳芳,李鑫,等.纳米BiOI 的稳定性、结构及光催化性能研究[J].有色金属科学与工程,2011,2(4):86-91. http://ysjskx.paperopen.com/oa/DArticle.aspx?type=view&id=20110420 [2] 余长林,杨凯.异质结构的复合光催化材料的研究新进展[J].有色金属科学与工程,2010,1(2):16-23. http://www.cnki.com.cn/Article/CJFDTOTAL-JXYS201006004.htm [3] Yu C L,Yu J C.A simple way to prepare C-N-codoped TiO2 photo- catalyst with visible light activity[J]. Catalysis Letters,2009,129(3/4): 462-470. http://cn.bing.com/academic/profile?id=2474811366&encoded=0&v=paper_preview&mkt=zh-cn
[4] 余长林, 操芳芳, 舒庆,等.Ag/BiOX (X=Cl, Br, I)复合光催化剂的制备、表征及其光催化性能[J].物理化学学报,2012,28(3): 647-653. [5] Cao Y Q,He T,Chen Y M,et al.Fabrication of rutile TiO2-Sn/Anatase TiO2-N heterostructure and its application in visible-light photocatal- ysis[J].The Journal of Physical Chemistry B,2010,114 (8):3627- 3633.
[6] Yu C L,Zhou W Q,Yang K,et al.Hydrothermal synthesis of hemi- sphere -like F -doped anatase TiO2 with visible light photocatalytic activity [J].Journal of Materials Science,2010,45(21):5756-5761. doi: 10.1007/s10853-010-4646-6
[7] Yu C L,Cai D J,Yang K,et al.Sol-gel derived S, I-codoped meso- porous TiO2 photocatalyst with high visible-light photocatalytic activi- ty [J].Journal of Physics and Chemistry of Solids,2010,71(9):1337- 1343. doi: 10.1016/j.jpcs.2010.06.001
[8] Yu C L,Yu J C,Chan M.Sonochemical fabrication of fluorinated mesoporous titanium dioxide microspheres[J]. Journal of Solid State Chemistry,2009,182(5):1061-1069. doi: 10.1016/j.jssc.2009.01.033
[9] 余长林,温和瑞,相彬,等.不同晶体结构的BiVO4 的制备及其可见光催化性能[J].江西理工大学学报,2009,30(4):9-12. http://www.cnki.com.cn/Article/CJFDTOTAL-NFYX200904006.htm [10] Yu C L,Meng X J.A novel Ag/BiOBr nanoplate catalyst with high photocatalytic activity in decomposition of the dyes [J].Reaction Ki- netics Mechanisms and Catalysis,2011,103(1):141-151. doi: 10.1007/s11144-011-0291-6
[11] Yu C L,Yu J C,Fan C F.Synthesis and characterization of Pt/BiOI nano-plate catalyst with enhanced activity under visible light irradi- ation[J].Materials Science and Engineering B, Solid-State Materials for Advanced Technology,2010,166(3):213-219. doi: 10.1016/j.mseb.2009.11.029
[12] Yu C L,Fan C F,Yu J C.Preparation of bismuth oxyiodides and ox- ides and their photooxidation characteristic under visible/UV -light irradiation [J].Materials Research Bulletin,2011,46(1):140-146. doi: 10.1016/j.materresbull.2010.08.013
[13] 张立德.纳米材料[M].北京:化学工业出版社,2000:37-39. [14] Henglein A.Small particle research: physicochemical properties of extremely small colloidal metal and semiconductor particles [J] . Chemical Reviews,1989,89(8):1861-1873. doi: 10.1021/cr00098a010
[15] Müller B R,Majoni S,Memming R,et al.Particle size and surface chemistry in photoelectrochemical reactions at semiconductor parti- cles [J].The Journal of Physical Chemistry B,1997,101(14):2501- 2507. doi: 10.1021/jp962749v
[16] Kanemoto M,Hosokawa H,Wada Y,et al.Semiconductor photocatal- ysis.Part 20.-Role of surface in the photoreduction of carbon dioxide catalysed by colloidal ZnS nanocrystallites in organic solvent[J].Jour- nal of the Chemical Society, Faraday Transactions,1996,92 (13): 2401-2411. doi: 10.1039/FT9969202401
[17] Qian Y T,Su Y,Xie Y,et al. Hydrothermal preparation and charac- terization of nanocrystalline powder of sphalerite[J]. Materials Research Bulletin,1995,30(5):601-605. doi: 10.1016/0025-5408(95)00040-2
[18] Feng S A,Zhao J H,Zhu Z P,et al.Kinetically restraining aggrega- tion of ZnS nanocrystals and the effect on photocatalysis [J].Materi- als Science and Engineering: B, Solid -State Materials for Ad- vanced Technology,2008,150(2):116-120. doi: 10.1016/j.mseb.2008.02.002
[19] Stroyuk A L,Raevskaya A E,Korzhak A V,et al.Zinc sulfide nanoparticles: spectral properties and photocatalytic activity in metals reduction reactions [J] . Journal of nanoparticle research , 2007,9(6):1027-1039. doi: 10.1007/s11051-006-9183-5
[20] Yu C L,Yu J C,Wen H R,et al.A mild solvothermal route for preparation of cubic-like CuInS2 crystals [J] . Materials Letters , 2009,63(23):1984-1986. doi: 10.1016/j.matlet.2009.06.030
[21] Yu C L,Wen H R,Yu J C.Preparation of different magnetic Fe3O4 nanocrystals under mild conditions with different poly (ethylene glycol) [J].Nanotechnology and Precision Engineering,2010,8 (2): 161-166. http://d.wanfangdata.com.cn/ExternalResource-nmjsyjmgc201006008%5e21.aspx
[22] 余长林,杨凯,范采凤,等.溶剂热合成Sn 掺杂的纳米ZnO 光催化剂及其光催化性能[J].纳米技术与精密工程,2011,9(6): 499-503. http://www.cnki.com.cn/Article/CJFDTOTAL-NMJM201106007.htm [23] 高濂,郑珊,张青红.纳米氧化钛光催化材料及应用[M].北京: 化学工业出版社,2002:72. [24] Suyver J F,Wuister S F,Kelly J J,et al.Synthesis and photolumines- cence of nanocrystalline ZnS:Mn2+[J].Nano Letters,2001,1(8):429- 443. doi: 10.1021/nl015551h
[25] 邓玲娟,黄方千,高丰琴,等.ZnS 光催化剂对不同偶氮类染料光降解的光催化性能的比较[J].应用化学,2010,27(6):705-709. http://www.cnki.com.cn/Article/CJFDTOTAL-YYHX201006021.htm




 下载:
下载: