On the characteristics of co-gasification of coals and biomass char
-
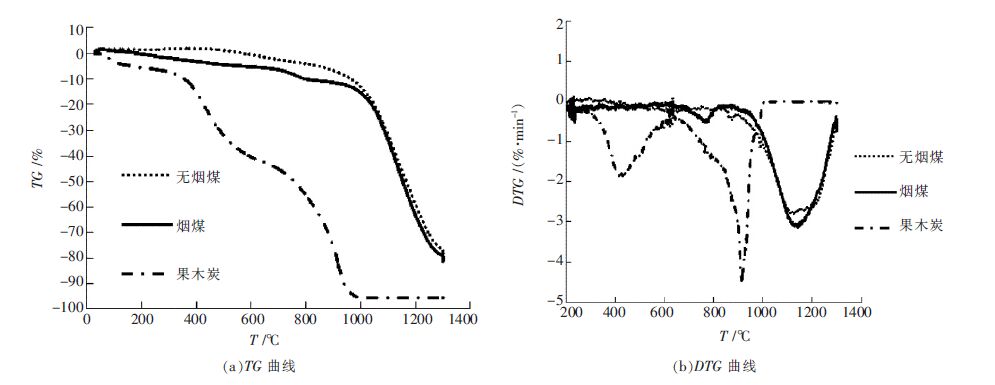
摘要: 本文利用非等温热重分析法研究了生物质焦与煤的共气化特性 ,探讨了生物质焦与煤共气化反应特征温度的变化情况, 并分别运用 Doyle 和 Coats-Redfern 近似函数计算了反应动力学参数. 采用 Doyle 法计算时,相关系数 R2 值比 Coats-Redfern 法更大,因此 Doyle 法计算结果更合理.采用 Doyle 法计算出: 生物质焦的气化反应活化能为 134.97 kJ/mol, 烟煤和无烟煤分别是 197.85 kJ/mol 和 171.36 kJ/mol.随着无烟煤 、烟煤中生物质焦掺混的比例增加 ,反应活化能不断下降.研究结果表明:生物质焦可以促进两种煤的气化反应,降低煤的最终反应温度和气化反应活化能.Abstract: This paper studies the co -gasification reaction of biomass char and coals by non -isothermal thermo gravimetric analysis. The change of the characteristic temperature of co-gasification reaction was investigated, while Doyle and Coats-Redfern approximate functions could be used to simulate kinetics parameters of the reaction. The data calculated by using Doyle method hold a higher value of R2 than the Coats -Redfern method. The Doyle method is more reasonable. In Doyle method, the apparent activation energy of the biomass char used is about 134.97 kJ/mol,while the bituminite is 197.85 kJ/mol and the anthracite is 171.36 kJ/mol. As the weight rate of biomass char added in coals become larger, the apparent activation energy become smaller. It was concluded that biomass char can make a promote role in coals' gasification process through lowing the final reaction temperature and the apparent activation energy.
-
Keywords:
- biomass char /
- coal /
- co-gasification /
- the apparent activation energy
-
0 前言
城门山铜矿1995年12月开始基建, 几经周折, 终于在2001年12月28日产出了合格的铜精矿, 在过去的一年半的时间里已产出铜金属量8 000多吨, 实现了城铜几代人的夙愿。城门山铜矿作为江铜集团的后备矿山之一, 在近两、三年内生产规模将由现在的39.6万t/ a扩大到90万t/ a。在生产规模逐步扩大的同时, 进一步查明矿床地质特征显得尤为重要, 特别是研究其表生变化过程及其次生富集作用, 对矿床开采具有重要的指导作用。
1 矿区地质概况
1.1 地质概况
城门山矿区位于九江市南77°西, 大地构造位置属长江中下游铜铁成矿带中部, 九瑞铜矿田的南东端。
矿区自南至北出露地层有志留、泥盆系砂岩及砂岩夹页岩, 石炭、二叠系灰岩及灰岩夹页岩, 三叠系页岩及第四系, 地层总体走向北东70°, 倾向北或北西, 倾角45~60°不等。构造主要有北东东向单斜和次级横跨褶皱, 北东东、北西及北北东三组断裂构成矿区构造格架, 控制岩体及矿体产出。岩浆岩主要为燕山期花岗闪长斑岩, 及少量的石英斑岩, 出露面积仅0.8km2。
城门山矿床分为3个矿带、5个亚带。铜硫矿体主要赋存在燕山期花岗闪长斑岩与石炭、二叠、三叠系碳酸盐岩的接触带上, 部分矿体产于花岗闪长斑岩体中, 工业铜硫矿体主要有108个, 其中铜金属量在5万t以上的矿体有9个, 占全区铜金属总量的95%以上, 以1#铜矿体规模最大。矿体形态主要有似层状、豆荚状、透镜状、带状及席状。矿石类型主要为含铜矽卡岩、含铜斑岩、含铜黄铁矿, 其次为含铜角砾岩、含铜褐铁矿等。已知矿石矿物达70余种, 其中金属矿物主要有黄铁矿、黄铜矿、褐铁矿、针铁矿、赤铁矿、辉铜矿、斑铜矿、孔雀石、蓝铜矿、和自然铜等, 脉石矿物主要有石英、石榴石、方解石、长石和高岭土等。
1.2 矿床自然分带特征
矿床自然分带明显, 根据钻孔样中氧化铜的含量与其全铜的比例、结合矿物组合特征, 划分出城门山矿区铜矿床矿石自然类型, 可分为氧化带、混合带和原生带3个带。
(1) 氧化带。全区氧化矿平均氧化率为37 %, 氧化矿石量占全区铜矿石总量的7.2%, 含铜品位0.93%, 铜金属量占8.9 %。常见标志矿物为孔雀石, 蓝铜矿、自然铜、赤铁矿、褐铁矿、氧化锰(多为硬锰矿)等为其特征。含铜矿物主要有孔雀石、蓝铜矿、自然铜、赤铜矿、黝铜矿、胆矾、及残留的黄铁矿、黄铜矿、辉铜矿、斑铜矿等组成。
(2) 混合带。全区混合矿平均氧化率为19 %, 混合带很发育, 该带铜金属量占全区铜矿石总量的33.2%, 含铜品位0.85%, 铜金属量占29.4 %。辉铜矿为该带标志矿物。含铜矿物以辉铜矿、黄铜矿、黄铁矿、斑铜矿、蓝辉铜矿、黝铜矿、孔雀石、蓝铜矿、自然铜等。
(3) 原生带。全区平均氧化率为5%, 原生矿石量占总全区铜矿石总量的63.3 %, 含铜品位0.69 %, 铜金属量占57.8%。含铜矿物以原生黄铜矿、斑铜矿、铜蓝、黝铜矿、含铜黄铁矿为主, 辉铜矿相对较少, 不见褐铁矿、孔雀石、自然铜等。
2 矿床的表生变化及其分带
表生变化指的是矿床的近地表和露出地表的部分, 在氧、地下水、碳酸气及风化等的长期作用下所发生的不同程度的变化。表生变化一般发生在矿床的氧化带与混合带的过渡带中。表生变化的结果会改变原矿床的组构、矿物成分和化学成分, 在金属硫化物矿床中表现得尤为强烈, 并可使某些金属在流动带中富集, 从而大大地提高矿床的工业价值。
城门山铜矿区三面环湖, 氧、地下水、湖水、碳酸气及风化等的长期作用下, 表生分带发育完整。综合矿区氧化带主要矿物空间分布特征, 表生分带大致可分为5个亚带(见表 1): ①完全氧化亚带-铁帽。②褐铁矿、孔雀石亚带。③次生氧化物富集亚带-蓝铜矿、孔雀石。④自然元素富集亚带-自然铜。⑤次生硫化物富集亚带。
表 1 城门山矿区铜矿床表生分带特征表 %
2.1 完全氧化亚带
位于氧化带的最上部, 氧化作用进行得最为强烈, 氧化时间也最长, 以铁的氧化物和氢氧化物占绝对优势, 故称为铁帽, 一般发育在零米标高以上。铁帽呈褐色至棕红色, 具松散或多孔状、蜂窝状构造。铁帽是金属硫化物矿床的重要标志。
2.2 褐铁矿、孔雀石亚带
直接出露地表, 一般发育在零米标高左右, 主要赋存在断层和破碎地段, 由褐铁矿、赤铁矿和下部的孔雀石等矿物组成。其化学反应过程为:

2.3 次生氧化物富集亚带
主要位于岩体内并在潜水面之上(0~-10m), 矿区只局部见及。主要由蓝铜矿、孔雀石等矿物组成。并见少量辉铜矿。其矿物形成主要在围岩为碳酸盐时, 黄铜矿氧化后的产物, 其化学反应过程为:

2.4 自然元素富集亚带
主要受地表破碎带(角砾岩)控制, 多分布于湖区(一般在-50~-90m左右)。主要矿物为自然铜, 并伴有贵金属自然金、自然银的富集, 亦可见赤铁矿。自然铜主要为已形成和原生辉铜矿氧化的结果。其化学反应过程为:

2.5 次生硫化物富集亚带
位于氧化带之下, 原生铜的硫化物常常被次生辉铜矿、蓝辉铜矿所交代, 使原生黄铜矿、黄铁矿矿石变成辉铜矿、黄铁矿矿石, 即次生富集铜矿石。该带上限在岩体内比较稳定(标高在0~-10m), 下限标高-50m, 最深达-150m, 其他区段为-10m~-50m左右标高。含铜矿物以辉铜矿为主, 少量蓝辉铜矿、铜蓝、斑铜矿与之相伴生。其化学反应过程为:

3 次生富集作用
在硫化物矿床的氧化带中淋漓出来的金属硫酸盐溶液, 当渗透到潜水面之下的还原环境中, 以交代原生硫化物的方式生成新的硫化矿物, 即为次生硫化物, 这样可以大幅度地提高矿石中的金属含量, 提高矿床的工业价值, 通常把这类富集金属的作用就称为次生富集作用, 发生此类作用的地带称为次生富集带。
矿区内铁帽发育广泛, 最深可达-260m, 氧化带中含有大量的褐铁矿、孔雀石、蓝铜矿等标型矿物。在氧、水、碳酸气等的长期作用下, 使原生铜的硫化物(主要为黄铜矿)转变为氧化物、氢氧化物、硫酸盐、碳酸盐。当铜的硫酸盐转溶液下渗至原生硫化物(黄铜矿)时, 在还原环境下产生次生富集作用, 形成大量的次生硫化矿物, 如辉铜矿、斑铜矿、铜蓝、黄铜矿等; 如潜水面不断变化, 即氧化、还原环境不断改变则又会发生新的变化, 或形成新矿物, 或形成硫酸铜又重新转入溶液, 这样反复交替进行, 其变化十分复杂。说明矿床经过次生富集作用后, 可大大地提高矿床的工业价值(其铜品位变化过程见表 2)。
表 2 城门山矿区次生富集作用过程分带特征表 %
矿区内次生富集现象多, 而且明显。现开采的7#铜矿体的氧化矿与混合矿过渡带中(标高一般在-10m以下), 次生富集作用也很明显, 而且形成的是高品位富铜矿石, 如7#铜矿体中的GK2 -43、GK 2 -44、GK43、CK2 -41、CK41等钻孔均见到了富矿段, 厚度在15~30m。如图 1所示GK43孔, 含铜品位在0.15 %~19.64 %, 次生富集带的厚度达25m以上, 为一富矿段, 具有很好的开采价值。10#铜矿体是由矿化围岩经次生富集作用形成的, 钻孔铜品位从0.28 %~1.85 %不等, 变化较大, 这是由于原生矿体或矿化岩石在不同基础上产生次生富集作用的结果; 10#铜矿体总的富集中心连线呈北东方向。在岩体中心, 斑岩矿化均匀, 品位较低, 迭加次生富集作用后, 原生矿化的品位不高; 从ZK411、ZK1038等钻孔的垂向上来看, 矿体上盘为星点状含铜褐铁矿的淋滤帽, 含铜0.1 %~0.2 %, 到矿体部分含铜增至0.5~5 %, 向下逐步降低到0.4 %~0.3 %, 再下为含铜小于0.2 %之正常矿化围岩, 铜含量变化明显, 是由于矿体经表生变化和迭加次生富集作用而形成的。
4 结语
城门山矿区地处赛城湖的南岸, 在氧、地下水、湖水、碳酸气及风化等的长期作用下, 矿床的表生变化比较复杂, 分带发育完整, 次生富集作用普遍, 常次生富集成富矿体、富矿段, 因此, 研究该矿床的表生分带过程及其次生富集作用, 圈定次生富集带, 对矿山企业生产具有重要指导意义。
-
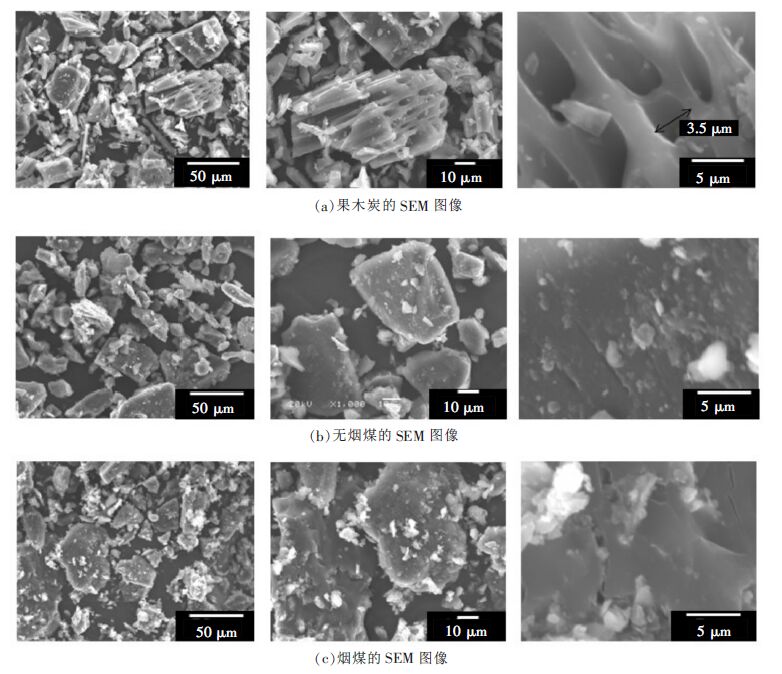
表 1 含碳材料的工业分析及灰分的主要成分/wt%

表 2 气化实验样品种类

表 3 3 种含碳材料的活化能

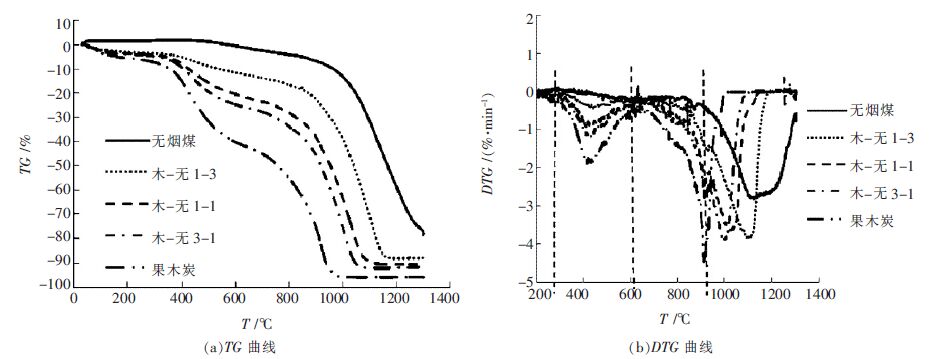
表 4 无烟煤与木炭共气化特性参数

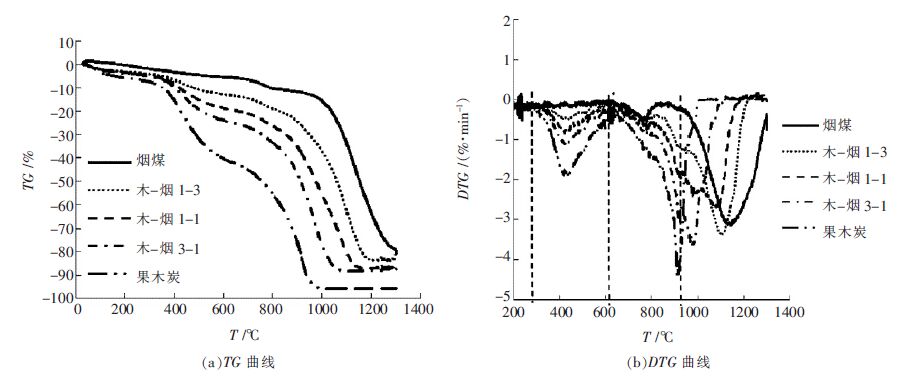
表 5 烟煤与木炭共气化特性参数

表 6 烟煤、无烟煤与果木炭混合时的气化反应活化能

-
[1] 梁卫平 .21 世纪生物质能研究 [J]. 科技情报开发与经济,2007,17 (4): 167-168. http://www.cnki.com.cn/Article/CJFDTOTAL-KJQB200704097.htm [2] 石元春.中国生物质原料资源 [J].中国工程科学,2011,13 (2): 16-23. http://www.cnki.com.cn/Article/CJFDTOTAL-GCKX201102004.htm [3] 董玉平,王理鹏,邓波,等. 国内外生物质能源开发利用技术 [J].山东大学学报 :工学版,2007,37 (3): 1-7. http://www.cnki.com.cn/Article/CJFDTOTAL-SDGY200703010.htm [4] 梁蒙. 生物质能源开发利用概述 [J].能源与环境,2010(33): 131. http://www.cnki.com.cn/Article/CJFDTOTAL-ZXLJ201033101.htm [5] 林兰英,陈志林,傅峰. 木材炭化与炭化物利用研究进展 [J]. 世界林业研究,2007,20(5): 22-26. http://www.cnki.com.cn/Article/CJFDTOTAL-SJLY200705005.htm [6] 李烨,吴幼青,顾菁,等. 秸秆焦的表面特征及 CO2 气化反应性的研究 [J]. 太阳能学报,2009,30 (9):1258-1263. http://www.cnki.com.cn/Article/CJFDTOTAL-TYLX200909022.htm [7] 杨海明,韩成利,吴也平,等. 生物质的热裂解 [J]. 高师理科学刊,2008,28(3):56-60. [8] 张科达,步学朋,王鹏,等. 生物质与煤在 CO2 气氛下共气化特性的初步研究 [J]. 煤炭转化,2009,32(3): 9-12. http://www.cnki.com.cn/Article/CJFDTOTAL-MTZH200903004.htm [9] Toshimitsu Suzuki,Hiroshi Nakajima. Effect of mineral matters in biomass on the gasification rate of their chars [J]. Biomass Conv. Bioref, 2011(1): 17-28. https://www.researchgate.net/publication/225837648_Effect_of_mineral_matters_in_biomass_on_the_gasification_rate_of_their_chars
[10] A. W. Coats,J. P. Redfern. Nature, 1964(201): 68.
[11] Vladimir Strezov. Re newable Energy, 2006, 31: 1892-1905.
[12] 刘忠锁,汪琦,邹宗树,等.非等温热重分析研究碳气化动力学 [J].材料与冶金学报,2010,9(1): 68-71. http://www.cnki.com.cn/Article/CJFDTOTAL-HUJI201001015.htm [13] 向军,陈伟,孙路石,等.典型煤种的二氧化碳气化特性研究[J]. 华中科技大学学报,2007,35(6): 89-91. http://www.cnki.com.cn/Article/CJFDTOTAL-HZLG200706027.htm



 下载:
下载: